As the global energy landscape shifts toward sustainable and green alternatives, the demand for efficient electrochemical energy storage devices has become paramount. Lithium-ion batteries have played a crucial role in portable electronics and electric vehicles, but safety concerns due to flammable electrolytes and limited energy density hinder their application in advanced fields like electric aviation and long-range transportation. In this context, all-solid-state lithium metal batteries (ASSLMBs) emerge as a promising next-generation technology, offering enhanced safety by replacing liquid electrolytes with solid-state counterparts and higher energy density through the use of lithium metal anodes. However, despite their theoretical advantages, ASSLMBs face significant challenges related to volume expansion during cycling, which induces complex electrochemo-mechanical coupling effects. These effects can lead to stress accumulation, interface degradation, and performance decay, ultimately limiting battery life and reliability. This article comprehensively examines the volume expansion phenomena in solid state batteries, explores their underlying mechanisms, and discusses strategies for mitigation, with a focus on material design, interface engineering, and structural optimization.
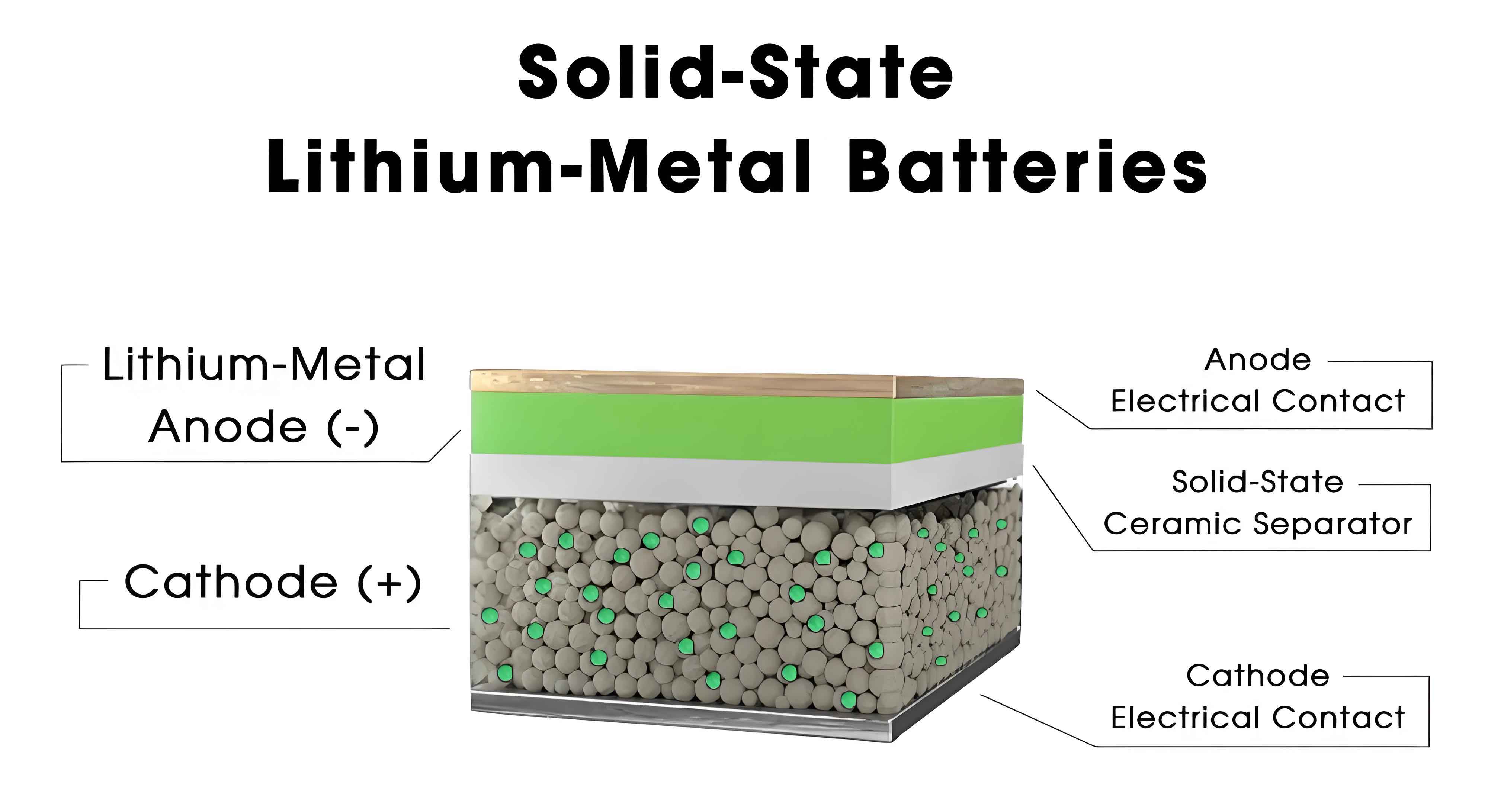
The transition to all-solid-state batteries is driven by their potential to overcome the limitations of conventional lithium-ion systems. Solid state batteries utilize solid electrolytes, which are non-flammable and can enable the integration of high-capacity lithium metal anodes. This combination promises higher energy densities and improved safety profiles. However, the rigid nature of solid-state components introduces mechanical challenges, as volume changes in electrode materials during charge and discharge cycles generate internal stresses. These stresses can cause contact loss, crack propagation, and dendrite formation, adversely affecting the performance and longevity of solid state batteries. Understanding and managing these volume expansion effects is critical for the practical deployment of all-solid-state lithium metal batteries.
Volume expansion in solid state batteries primarily arises from the lithiation and delithiation processes in electrode materials. For instance, cathode materials like nickel-cobalt-manganese (NCM) oxides undergo lattice parameter changes, leading to volume variations of 2–10%. In contrast, lithium metal anodes can experience extreme volume expansion, up to 1000% under certain conditions. This disparity in volume changes between electrodes exacerbates the mechanical strain within the battery cell. In high-energy-density configurations, such as those targeting over 600 W·h/kg, the overall cell volume change can exceed 18%, far beyond the tolerable limits for conventional battery designs. The resulting stresses, which can range from MPa to GPa levels, influence ion transport, reaction kinetics, and interface stability, creating a feedback loop that impacts the electrochemical performance of solid state batteries.
To quantify the volume changes in solid state batteries, we can analyze the contributions from various components. The following table summarizes the volume expansion rates for common electrode materials used in all-solid-state lithium metal batteries:
| Material Type | Specific Capacity (mA·h/g) | Volume Change Rate (%) |
|---|---|---|
| NCM Cathode | 180–220 | 2–10 |
| LFP Cathode | 150–170 | ~2 |
| Lithium Metal Anode | 3860 | Up to 1000 |
| Graphite Anode | 372 | ~10 |
| Silicon Anode | 4200 | ~300 |
The electrochemo-mechanical coupling in solid state batteries can be described through free energy considerations. The total free energy density ψ of the system includes chemical, mechanical, and coupling terms:
$$ \psi = \psi_{\text{chem}}(c) + \psi_{\text{mech}}(\varepsilon) + \psi_{\text{coupling}}(c, \varepsilon) $$
Here, ψchem(c) represents the chemical free energy dependent on ion concentration c, ψmech(ε) accounts for mechanical energy due to strain ε, and ψcoupling(c, ε) captures the interaction between concentration and strain. This coupling leads to stress generation during ion insertion or extraction, which in turn affects the chemical potential μ:
$$ \mu = \mu_0 + RT \ln a + V \sigma $$
where μ0 is the standard chemical potential, R is the gas constant, T is temperature, a is ion activity, V is molar volume, and σ is stress. In solid state batteries, this relationship implies that stress fields can alter ion migration paths and reaction rates, influencing the overall performance of all-solid-state lithium metal batteries.
At the cell level, volume expansion manifests as overall thickness changes during cycling. For example, in a solid state battery with a lithium-rich manganese oxide cathode and lithium metal anode, the anode’s volume expansion dominates the cell’s dimensional changes. The strain rate due to lithium deposition can be expressed as:
$$ \dot{\varepsilon} = \frac{J A}{h F N} $$
where J is current density, A is area, h is thickness, F is Faraday’s constant, and N is the number of moles. This strain induces stress, particularly under constrained conditions, leading to creep deformation in lithium metal. The power-law creep model describes this behavior:
$$ \dot{\varepsilon}_c = A_c \sigma^n \exp\left(-\frac{Q_c}{RT}\right) $$
where Ac is a material constant, n is the stress exponent, and Qc is activation energy. For lithium metal, n is approximately 6.4, indicating high stress sensitivity. In practical solid state batteries, this can result in significant stack pressures, necessitating robust module designs to mitigate mechanical failure.
Localized volume changes pose additional challenges in all-solid-state lithium metal batteries. Lithium dendrite growth, for instance, occurs due to non-uniform current distribution and interface defects. The stress at dendrite tips can be estimated by:
$$ \sigma = \frac{F \Delta \phi}{V_m} $$
where Δφ is the overpotential and Vm is the molar volume of lithium. Even small overpotentials can generate high stresses, promoting crack propagation in solid electrolytes. Similarly, lithium void formation during stripping cycles leads to local volume contraction, disrupting interface contact and increasing impedance. The strain rate for void-induced contraction is given by:
$$ \dot{\varepsilon} = \frac{J M}{F h \rho} $$
where M is molar mass and ρ is density. High current densities accelerate void growth, exacerbating performance degradation in solid state batteries.
On the cathode side, active material particles experience anisotropic volume changes and concentration gradients during cycling. For example, NCM particles exhibit variations in lattice parameters a and c, causing internal stresses that lead to cracking. The stress intensity factor K for crack propagation is:
$$ K = \sigma \sqrt{\pi \gamma \alpha} $$
where γ is a geometric factor and α is defect size. Phase transitions, such as those induced by oxygen release, further complicate volume changes, contributing to structural degradation in all-solid-state lithium metal batteries.
To address these issues, various mitigation strategies have been developed for solid state batteries. Module-level approaches include incorporating elastic components like springs or foams to absorb expansion-induced stresses. Studies show that such designs can reduce peak stresses by over 20% and extend battery life. Material-level strategies focus on developing zero-strain electrodes, such as alloy anodes and doped cathodes, which minimize volume changes. For instance, lithium-magnesium alloys exhibit reduced expansion due to stable phase transformations, while aluminum-doped NCM cathodes show lower volume variation. Interface engineering, through artificial layers or composite structures, enhances contact and suppresses dendrite growth. The following table summarizes key mitigation techniques for volume expansion in solid state batteries:
| Strategy Category | Approach | Impact on Volume Expansion |
|---|---|---|
| Module Design | Elastic buffering with springs/foams | Reduces stack pressure by 20–30% |
| Anode Modification | 3D host structures or alloy formation | Limits expansion to <50% |
| Cathode Optimization | Single-crystal particles or doping | Decreases volume change to 1–2% |
| Interface Engineering | Conductive interlayers or coatings | Prevents localized stress concentration |
For lithium dendrite suppression in solid state batteries, approaches include enhancing lithium diffusion kinetics and inducing directional deposition. Composite anodes with fast ion conductors, such as Li3N or carbon matrices, improve lithium transport, reducing local stress. The critical current density Jc for dendrite initiation can be modeled as:
$$ J_c = \frac{D F c_0}{L} $$
where D is diffusion coefficient, c0 is initial concentration, and L is diffusion length. By optimizing these parameters, solid state batteries can achieve higher rate capabilities and longer cycle life.
In cathode composites, blending materials with complementary volume changes can offset overall expansion. For example, combining NCM (positive volume change) with LCO (negative volume change) reduces the net strain in all-solid-state lithium metal batteries. Finite element simulations demonstrate that such mixtures can lower stress by up to 50% compared to single-material electrodes.
Looking ahead, future research on solid state batteries should prioritize multi-physics modeling to integrate electrochemical, thermal, and mechanical phenomena. Advanced characterization techniques, such as in-situ stress mapping and high-resolution tomography, will provide deeper insights into volume expansion dynamics. Machine learning can accelerate material discovery and design optimization for all-solid-state lithium metal batteries. Additionally, developing scalable manufacturing processes and standardized testing protocols is essential for commercial adoption. By addressing the electrochemo-mechanical challenges, solid state batteries can realize their full potential as safe, high-energy-density storage solutions.
In summary, volume expansion in all-solid-state lithium metal batteries is a critical issue that requires comprehensive understanding and innovative solutions. Through material advances, interface control, and structural design, the detrimental effects of volume changes can be mitigated, paving the way for the widespread application of solid state batteries in next-generation energy systems.
