With the rapid expansion of lithium-ion batteries in consumer electronics, electric vehicles, and energy storage systems, there is a growing demand for battery technologies that offer higher energy density and improved safety. However, conventional lithium-ion batteries utilizing liquid electrolytes face limitations such as insufficient stability at high voltages, risks of leakage, and dendrite growth leading to short circuits. Solid-state batteries, which incorporate solid electrolytes, present a promising solution to these challenges. Recent developments in high-ionic-conductivity solid electrolytes, optimization of solid-solid interfaces, and research into composite electrode materials have accelerated the progress of solid-state batteries. Despite these advancements, issues like lithium-ion transport barriers between phases and high interfacial impedance continue to hinder their commercialization. In this review, we propose lithium-ion transport throughput as a critical metric for evaluating the comprehensive performance of solid-state batteries. We comprehensively assess strategies to enhance this throughput, focusing on bulk ion transport in solid electrolytes, electrode/electrolyte interface design, and synergistic ion/electron transport networks within electrodes. Future efforts should integrate material design and structural optimization to improve the overall performance of solid-state battery systems, paving the way for the industrial development of high-safety and high-energy-density solid-state batteries.
The concept of lithium-ion transport throughput, denoted as $\Phi_{Li^+}$, quantifies the number of moles of lithium ions passing through a unit area of the electrode/electrolyte interface per hour during charge or discharge reactions. It is calculated using the formula:
$$\Phi_{Li^+} = \frac{10000 \times C_{\text{area}}}{C_{Li} \cdot M_{Li} \cdot t}$$
where $C_{\text{area}}$ is the areal specific capacity in mA·h·cm⁻², $C_{Li}$ is the theoretical specific capacity of lithium metal (3860 mA·h·g⁻¹), $M_{Li}$ is the molar mass of lithium (6.941 g·mol⁻¹), and $t$ is the charge or discharge time in hours. This metric integrates areal capacity and charge-discharge rate, providing a more accurate depiction of internal electrochemical processes than external parameters like current density. For instance, recent studies on solid-state batteries have reported $\Phi_{Li^+}$ values ranging from 0.038 to 1.977 mol·m⁻²·h⁻¹, whereas liquid electrolyte-based lithium-metal batteries can achieve up to 2.799 mol·m⁻²·h⁻¹, highlighting the performance gap and potential for improvement in solid-state systems.
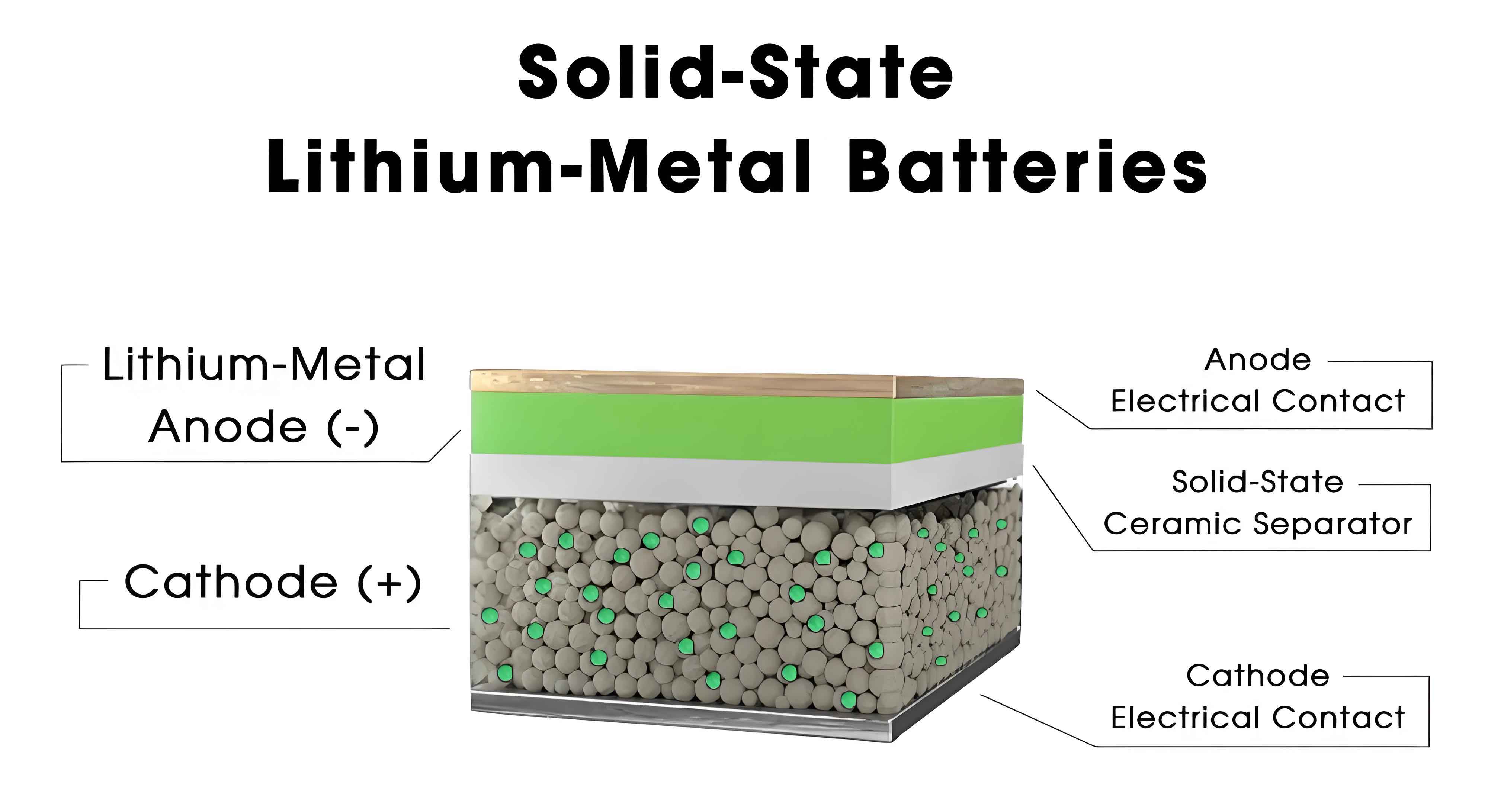
To enhance lithium-ion transport throughput in solid-state batteries, we focus on three primary strategies: improving bulk ion transport in solid electrolytes, optimizing interfacial ion transport at electrode/electrolyte interfaces, and enhancing synergistic ion and electron transport within thick, dense electrodes. Each of these areas addresses key bottlenecks in solid-state battery performance.
Enhancing Bulk Ion Transport in Solid Electrolytes
The ionic conductivity of solid electrolytes is fundamental to achieving high lithium-ion transport throughput in solid-state batteries. Inorganic solid electrolytes, such as oxides, sulfides, and halides, typically exhibit ionic conductivities above $10^{-4}$ S·cm⁻¹, but they often fall short of the $10^{-2}$ S·cm⁻¹ seen in liquid electrolytes. Ion transport in these materials relies on lithium ions hopping between sites via thermal vibration, influenced by factors like anion frameworks, non-lithium cation arrangements, and lithium site occupancy. Strategies to enhance ionic conductivity include introducing high-entropy structures to create local disorder, designing amorphous or glassy phases to eliminate grain boundaries, and engineering vacancies to facilitate ion migration.
For example, high-entropy doping in sulfide solid electrolytes has led to room-temperature ionic conductivities as high as 32 mS·cm⁻¹. The formula for a high-entropy sulfide electrolyte can be represented as Li$_9.54$[Si$_{0.6}$Ge$_{0.4}$]$_{1.74}$P$_{1.44}$S$_{11.1}$Br$_{0.3}$O$_{0.6}$, which demonstrates how multi-element incorporation increases configurational entropy and reduces energy barriers for ion migration. Similarly, in halide solid electrolytes, high-entropy compositions like Li$_{2.75}$Y$_{0.16}$Er$_{0.16}$Yb$_{0.16}$In$_{0.25}$Zr$_{0.25}$Cl$_6$ exhibit improved ionic conductivity and lower activation energies due to local lattice distortions. Amorphous solid electrolytes, such as those in the Li-Ta-Cl system, achieve conductivities up to 7.16 mS·cm⁻¹ by forming short-range ordered structures with abundant vacancies. The ionic conductivity $\sigma$ can be expressed using the Nernst-Einstein relation:
$$\sigma = \frac{n q^2 D}{k_B T}$$
where $n$ is the carrier concentration, $q$ is the charge, $D$ is the diffusion coefficient, $k_B$ is Boltzmann’s constant, and $T$ is temperature. Enhancing $D$ through structural modifications is key to improving $\sigma$.
Polymer-based solid electrolytes, while offering good flexibility and interface compatibility, suffer from low room-temperature ionic conductivities due to limited chain segment mobility. Composite solid electrolytes that blend inorganic fillers with polymers can leverage the high conductivity of inorganics and the flexibility of polymers. For instance, when inorganic fillers like LLZO form a percolating network in a PEO matrix, lithium ions primarily transport through the inorganic phase, boosting overall conductivity. The effective conductivity $\sigma_{\text{eff}}$ of a composite can be modeled using effective medium theory:
$$\sigma_{\text{eff}} = \sigma_m \left( \frac{\phi_f (\sigma_f – \sigma_m)}{\sigma_m + n (\sigma_f – \sigma_m)} \right)$$
where $\sigma_m$ and $\sigma_f$ are the matrix and filler conductivities, $\phi_f$ is the filler volume fraction, and $n$ is a shape factor. Advanced characterization techniques, such as solid-state NMR and synchrotron X-ray diffraction, have been instrumental in elucidating ion transport mechanisms in these composites.
| Electrolyte Type | Composition | Ionic Conductivity (S·cm⁻¹) | Reference |
|---|---|---|---|
| Sulfide | Li$_9.54$[Si$_{0.6}$Ge$_{0.4}$]$_{1.74}$P$_{1.44}$S$_{11.1}$Br$_{0.3}$O$_{0.6}$ | 3.2 × 10⁻² | High-entropy study |
| Halide | Li$_{2.75}$Y$_{0.16}$Er$_{0.16}$Yb$_{0.16}$In$_{0.25}$Zr$_{0.25}$Cl$_6$ | 1.04 × 10⁻³ | Halide optimization |
| Amorphous | Li-Ta-Cl based | 7.16 × 10⁻³ | Amorphous system |
| Polymer Composite | PEO-LLZO (50 wt%) | 2.26 × 10⁻⁴ | Composite design |
In summary, advancing bulk ion transport in solid electrolytes through high-entropy engineering, amorphous phase design, and composite strategies is crucial for increasing lithium-ion transport throughput in solid-state batteries. These approaches directly impact the foundational ion conduction capabilities of solid-state battery systems.
Optimizing Interfacial Ion Transport at Electrode/Electrolyte Interfaces
Interfacial ion transport is a critical challenge in solid-state batteries due to poor solid-solid contact, which leads to high impedance and limited lithium-ion transport throughput. The energy barrier for ion transport across interfaces is often higher than that for bulk transport, necessitating strategies to improve interface compatibility and stability. Key approaches include designing porous interfacial layers, introducing mixed ionic-electronic conductive interlayers, and forming alloy layers to enhance wettability and reduce resistance.
Porous interfacial layers, such as mixed ion-electron conducting (MIEC) structures in garnet electrolytes, facilitate uniform potential distribution and stress relief, enabling critical current densities up to 100 mA·cm⁻² and $\Phi_{Li^+}$ values of 0.69 mol·m⁻²·h⁻¹. The interfacial resistance $R_{\text{int}}$ can be described by:
$$R_{\text{int}} = \frac{\delta}{\sigma_{\text{int}}}$$
where $\delta$ is the interfacial layer thickness and $\sigma_{\text{int}}$ is its ionic conductivity. Minimizing $\delta$ while maximizing $\sigma_{\text{int}}$ is essential for reducing $R_{\text{int}}$.
Mixed conductive interlayers, such as Li$_7$N$_2$I-carbon nanotube composites, combine high ionic conductivity ($3.1 \times 10^{-4}$ S·cm⁻¹) with electronic conductivity to guide lithium deposition and prevent dendrite formation. These interlayers have achieved $\Phi_{Li^+}$ values of 0.44 mol·m⁻²·h⁻¹. Alloy-based interlayers, like Mg-Bi or Al-Si, react with lithium to form stable compounds (e.g., Li$_3$Bi or Li-Al alloys) that improve interface wettability and cycling stability. For example, Mg-Bi interlayers in LPSC electrolytes enable stable operation at 6.0 mA·cm⁻² for over 1798 hours, with $\Phi_{Li^+}$ reaching 0.939 mol·m⁻²·h⁻¹ in full cells.
On the cathode side, interface layers such as LiI or LiNbO$_3$ coatings suppress electrolyte decomposition and promote ion diffusion. The space charge layer effect at cathode/electrolyte interfaces can be mitigated by introducing functional layers that balance ion and electron transport. The space charge potential $\phi$ is given by Poisson’s equation:
$$\frac{d^2\phi}{dx^2} = -\frac{\rho(x)}{\epsilon}$$
where $\rho(x)$ is the charge density and $\epsilon$ is the permittivity. Designing interfaces with tailored charge distributions can minimize $\phi$ and enhance ion transport.
| Interface Strategy | Material System | $\Phi_{Li^+}$ (mol·m⁻²·h⁻¹) | Key Achievement |
|---|---|---|---|
| Porous MIEC Layer | Ta-LLZO with MIEC | 0.692 | Critical current density of 100 mA·cm⁻² |
| Mixed Conductive Interlayer | Li$_7$N$_2$I-CNT | 0.443 | Dendrite suppression |
| Alloy Interlayer | Mg-Bi on LPSC | 0.939 | Stable cycling at 6 mA·cm⁻² |
| Cathode Coating | LiNbO$_3$ on NCM | 0.037 | Enhanced interface stability |
In conclusion, optimizing interfacial ion transport through porous layers, mixed conductive interlayers, and alloy formations significantly boosts lithium-ion transport throughput in solid-state batteries. These strategies address the inherent solid-solid contact issues and are vital for achieving high-performance solid-state battery systems.
Enhancing Synergistic Ion and Electron Transport in Thick Electrodes
Thick electrodes with high active material loadings are essential for achieving high energy density in solid-state batteries, but they often suffer from inefficient ion and electron transport, leading to low lithium-ion transport throughput. The tortuous pathways in dense electrodes result in non-uniform current distribution and limited active material utilization. Strategies to overcome this include constructing integrated ion-electron conductive networks, designing aligned structures, and employing magnetic field-assisted orientation of conductive fillers.
Integrated conductive networks, such as “solid-polymer-solid” elastic channels using La$_2$Zr$_2$O$_7$ nanowires in PEO-based cathodes, enhance ion transport by providing continuous pathways. These networks have demonstrated $\Phi_{Li^+}$ values of 0.28 mol·m⁻²·h⁻¹ at 3 C rates. The effective tortuosity $\tau$ of an electrode can be reduced by aligning conductive materials, as described by:
$$\tau = \frac{L_e}{L}$$
where $L_e$ is the effective path length and $L$ is the electrode thickness. Lowering $\tau$ improves ion accessibility and increases $\Phi_{Li^+}$.
Aligned structures, such as arrayed or vasculated electrode designs, optimize ion and electron transport by creating dedicated pathways. For example, arrayed LiFePO$_4$ electrodes with PEO and CNT additives achieve $\Phi_{Li^+}$ of 0.87 mol·m⁻²·h⁻¹ at 1 C with areal capacities of 4.65 mA·h·cm⁻². Magnetic field-oriented LLTO nanowires in cathodes enable efficient ion percolation, resulting in $\Phi_{Li^+}$ of 0.11 mol·m⁻²·h⁻¹ at 0.1 C for high-loading electrodes.
On the anode side, composite lithium metals, such as Li-Mg-graphite ternary systems, improve ion diffusion and interface stability. These composites exhibit $\Phi_{Li^+}$ of 0.10 mol·m⁻²·h⁻¹ at 0.5 C by facilitating uniform lithium deposition and reducing void formation. The diffusion coefficient $D$ in composites can be enhanced by incorporating conductive additives, following the relation:
$$D = D_0 \exp\left(-\frac{E_a}{k_B T}\right)$$
where $D_0$ is the pre-exponential factor and $E_a$ is the activation energy. Lowering $E_a$ through material design accelerates ion transport.
Additionally,一体化 electrode-electrolyte designs, where the electrolyte also serves as an active material (e.g., Li$_3$TiCl$_6$ or Li$_3$VCl$_6$), eliminate interface resistance and enable $\Phi_{Li^+}$ values up to 0.062 mol·m⁻²·h⁻¹. These systems leverage the dual functionality of materials to simplify cell architecture and enhance throughput.
| Electrode Design | Material System | Areal Capacity (mA·h·cm⁻²) | $\Phi_{Li^+}$ (mol·m⁻²·h⁻¹) |
|---|---|---|---|
| Integrated Network | LZON-PEO in NCM811 | 0.260 | 0.485 |
| Aligned Structure | Arrayed LiFePO$_4$ with CNT | 4.65 | 0.870 |
| Magnetic-Oriented | LLTO nanowires in LFP | 3.00 | 0.112 |
| Composite Anode | Li-Mg-graphite | 0.539 | 0.101 |
In summary, enhancing synergistic ion and electron transport in thick electrodes through conductive networks, aligned structures, and composite designs is pivotal for increasing lithium-ion transport throughput in solid-state batteries. These approaches ensure efficient utilization of active materials and support the development of high-loading electrode systems.
Conclusion and Future Perspectives
Solid-state batteries represent a transformative technology for next-generation energy storage, offering advantages in safety and energy density. However, achieving high lithium-ion transport throughput remains a challenge due to limitations in bulk ion transport, interfacial impedance, and electrode-level transport inefficiencies. The metric $\Phi_{Li^+}$ provides a comprehensive means to evaluate and guide improvements in solid-state battery performance.
Future research should focus on integrating material innovations with system-level optimization. Key directions include:
- Developing novel solid electrolytes with higher ionic conductivities, lower costs, and environmental friendliness. High-entropy and amorphous materials show particular promise.
- Advancing interface engineering to ensure long-term stability and low impedance, using techniques like in-situ polymerization and atomic layer deposition.
- Optimizing electrode architectures to minimize tortuosity and maximize ion/electron synergy, potentially through 3D printing or field-assisted assembly.
- Leveraging advanced characterization methods, such as in-situ spectroscopy and machine learning, to unravel ion transport mechanisms and accelerate material discovery.
By addressing these aspects, solid-state batteries can achieve the high lithium-ion transport throughput necessary for commercial applications in electric vehicles and grid storage. The continued evolution of solid-state battery technology will play a crucial role in the global transition to sustainable energy and carbon neutrality.
