As a researcher in the field of energy storage, I have witnessed the rapid evolution of solid state batteries, which are poised to revolutionize the landscape of lithium-ion technology. The transition from liquid to solid electrolytes addresses critical safety concerns while unlocking higher energy densities. In this comprehensive review, I will delve into the application performance of electrolyte materials for solid state batteries, focusing on ionic conduction mechanisms, structural modifications, and industrial scalability. Solid state batteries represent a paradigm shift, and their success hinges on the development of robust solid electrolytes. Throughout this discussion, I will emphasize the importance of ionic conductivity, electrochemical stability, and interfacial compatibility in advancing solid state battery technology.
The fundamental challenge in solid state batteries lies in achieving high ionic conductivity in solid electrolytes, which traditionally lag behind their liquid counterparts. Solid electrolytes can be categorized into inorganic solid electrolytes (ISE), polymer solid electrolytes (PSE), and composite solid electrolytes (CSE). Each class exhibits distinct Li+ transport mechanisms. In ISEs, Li+ migration occurs via vacancy, interstitial, or cooperative mechanisms, often described by Arrhenius-type behavior: $$\sigma = \sigma_0 \exp\left(-\frac{E_a}{kT}\right)$$ where $\sigma$ is the ionic conductivity, $\sigma_0$ is the pre-exponential factor, $E_a$ is the activation energy, $k$ is Boltzmann’s constant, and $T$ is the temperature. For solid state batteries to achieve commercial viability, optimizing these parameters is crucial.
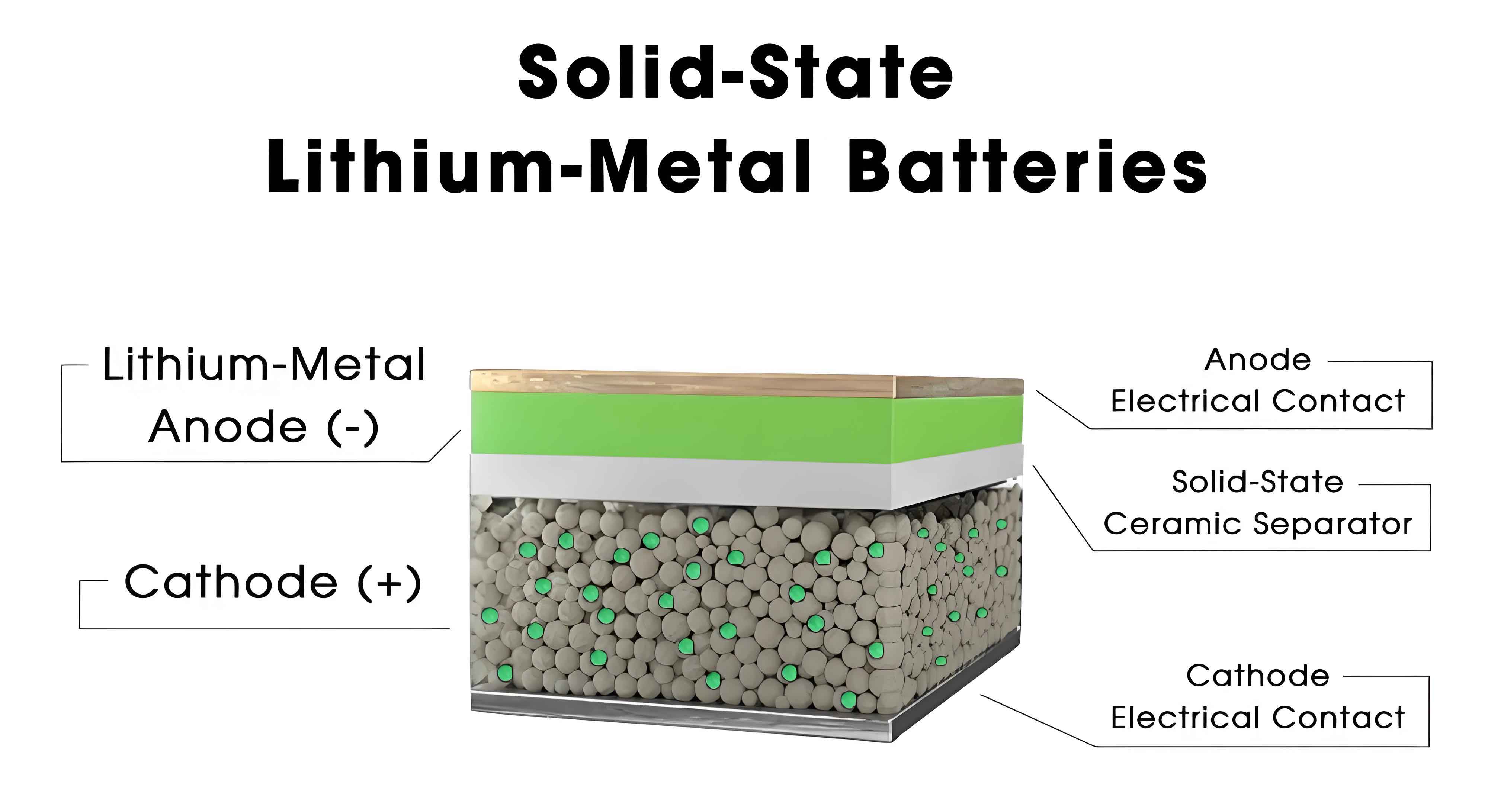
Inorganic solid electrolytes are renowned for their high ionic conductivity and stability. Among them, oxide-based solid electrolytes (OSE) like garnet-type Li7La3Zr2O12 (LLZO) and NASICON-type Li1.3Al0.3Ti1.7(PO4)3 (LATP) have been extensively studied. The Li+ transport in OSEs often involves vacancy-mediated diffusion, where doping with aliovalent ions enhances conductivity by creating vacancies. For instance, Ta5+ doping in LLZO stabilizes the cubic phase and increases Li+ mobility. The ionic conductivity can be modeled using the Nernst-Einstein relation: $$D = \frac{\sigma kT}{nq^2}$$ where $D$ is the diffusion coefficient, $n$ is the charge carrier density, and $q$ is the charge. Sulfide-based solid electrolytes (SSE), such as Li10GeP2S12 (LGPS), exhibit exceptional room-temperature conductivity exceeding 10−2 S/cm, but suffer from hygroscopicity. Halide-based solid electrolytes (HSE), like Li3InCl6, offer a balance of conductivity and stability, though cost remains a barrier. The performance of various ISEs is summarized in Table 1.
| Type | Representative Material | Ionic Conductivity (S/cm) | Activation Energy (eV) | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Oxide (OSE) | Li7La3Zr2O12 | 10−4 to 10−3 | 0.2–0.4 | High stability, wide window | Brittle, high sintering temperature |
| Sulfide (SSE) | Li10GeP2S12 | ~10−2 | 0.2–0.3 | High conductivity, deformable | Moisture sensitivity, narrow window |
| Halide (HSE) | Li3InCl6 | 10−3 to 10−4 | 0.3–0.4 | Good compatibility, moderate conductivity | Costly, poor Li stability |
Polymer solid electrolytes, such as those based on poly(ethylene oxide) (PEO), offer flexibility and ease of processing, which are advantageous for solid state batteries. However, their low room-temperature ionic conductivity (10−7 to 10−5 S/cm) limits application. The Li+ transport in PSEs relies on segmental motion of polymer chains above the glass transition temperature ($T_g$). The conductivity follows the Vogel-Tammann-Fulcher equation: $$\sigma = \sigma_0 \exp\left[-\frac{B}{T – T_0}\right]$$ where $B$ is a constant and $T_0$ is the ideal glass transition temperature. To enhance performance, blending with plasticizers or incorporating fillers is common. For example, PEO-based electrolytes with LiTFSI salt can achieve conductivities of ~10−4 S/cm at elevated temperatures. The mechanical properties, however, remain a challenge for inhibiting Li dendrite growth in solid state batteries. Table 2 compares different polymer systems.
| Polymer Matrix | $T_g$ (°C) | Melting Point (°C) | Ionic Conductivity (S/cm) | Key Features |
|---|---|---|---|---|
| PEO | −64 | 65 | 10−6 to 10−4 | Flexible, good Li transport |
| PVDF | −35 | 171 | 10−5 to 10−4 | High dielectric constant, stable |
| PAN | 125 | 317 | 10−6 to 10−5 | Thermal stability, wide window |
| PMMA | 105 | 150 | 10−5 to 10−4 | Low interface resistance, cost-effective |
Composite solid electrolytes merge the benefits of ISEs and PSEs, creating hybrid materials with enhanced performance for solid state batteries. By dispersing inorganic fillers like LLZO or Li6PS5Cl into polymer matrices, ionic conductivity can be boosted to 10−4–10−3 S/cm. The Li+ transport in CSEs occurs through multiple pathways: via the polymer phase, the filler particles, and interfacial regions. The effective conductivity can be estimated using percolation theory: $$\sigma_{\text{eff}} = \sigma_m (p – p_c)^t$$ where $\sigma_m$ is the matrix conductivity, $p$ is the filler volume fraction, $p_c$ is the percolation threshold, and $t$ is a critical exponent. Active fillers that conduct Li+ themselves, such as doped LLZO, are particularly effective. For instance, PEO-LiTFSI with 60% LLZO filler achieves a conductivity of 3.57 × 10−4 S/cm and a wide electrochemical window of 5.1 V. Such CSEs also improve mechanical strength, crucial for suppressing dendrites in solid state batteries. Table 3 highlights the performance of select CSEs.
| Composition | Ionic Conductivity (S/cm) | Li+ Transference Number | Electrochemical Window (V) | Cycling Stability |
|---|---|---|---|---|
| PEO-LiTFSI-60% LLZO | 3.57 × 10−4 | — | 5.1 | 97.26% capacity retention after 180 cycles |
| PVDF/Li3InCl6-15% | — | — | — | 82.8% retention after 200 cycles |
| PCL-3% Li6PS5Cl | 1.36 × 10−4 | 0.508 | 5.3 | >80% retention after 400 cycles |
Modification strategies play a pivotal role in enhancing solid electrolytes for solid state batteries. Doping with elements like Ta, Ga, or F can alter crystal structures, reduce activation energies, and improve ionic conductivities. For example, in LLZO, co-doping with Ga and Y lowers $E_a$ to 0.23 eV and increases conductivity to 9.95 × 10−4 S/cm. Similarly, F-doping in LATP via sol-gel methods raises conductivity to 3.58 × 10−4 S/cm by facilitating Li+ migration through lowered energy barriers. The defect chemistry involved can be described by Kröger-Vink notation: $$\text{Li}_\text{Li}^\times + \text{V}_\text{Li}’ \rightarrow \text{Li}_\text{i}^\bullet$$ where vacancies and interstitials govern diffusion. Moreover, advanced synthesis techniques, such as microwave-assisted hydrothermal methods or high-pressure low-temperature processing, enable denser microstructures and higher conductivities. These innovations are essential for scaling up solid state batteries.
From an industrial perspective, the commercialization of solid state batteries is accelerating globally. Companies are investing in sulfide, oxide, and polymer-based electrolytes to overcome existing limitations. For instance, sulfide electrolytes are favored for their high conductivity, while oxides offer better stability. The development of solid state batteries is not just a scientific endeavor but a strategic imperative for next-generation energy storage. However, challenges like interface resistance, dendrite suppression, and cost-effective manufacturing remain. Future research should focus on machine learning-guided material discovery and multi-scale modeling to optimize solid electrolytes. In conclusion, the progress in solid electrolyte materials is driving the evolution of solid state batteries, promising safer, higher-energy-density power sources. As I reflect on these advances, it is clear that interdisciplinary efforts will be key to realizing the full potential of solid state batteries.
In summary, the journey toward optimal solid electrolytes for solid state batteries involves a deep understanding of ion transport mechanisms, creative material engineering, and scalable production methods. The continuous improvement in ionic conductivity, electrochemical stability, and mechanical properties will undoubtedly propel solid state batteries into mainstream applications. I am optimistic that with sustained innovation, solid state batteries will soon overcome current hurdles and redefine energy storage for decades to come.
