In recent years, the demand for high-performance energy storage systems has surged, driven by advancements in electric vehicles, portable electronics, and renewable energy integration. Conventional liquid lithium-ion batteries have long dominated the market, but they suffer from inherent safety risks due to the use of flammable organic electrolytes. These risks include leakage, corrosion, combustion, and explosion under conditions such as overcharging, short-circuiting, or thermal runaway. In contrast, solid-state batteries utilize a solid electrolyte membrane, which offers superior safety by preventing vaporization, deformation, and internal short circuits even at elevated temperatures. This fundamental advantage positions solid-state batteries as a promising alternative, particularly for applications requiring long cycle life and enhanced reliability.
Our research focuses on long-cycle solid-state batteries, which exhibit exceptional performance metrics, including a gravimetric energy density of up to 350 Wh/kg, a capacity retention of over 80% after 1,200 cycles, and robust operation across a wide temperature range from -20°C to 80°C. These characteristics far surpass those of traditional liquid lithium-ion batteries, which typically achieve around 800 cycles and face significant degradation at temperatures above 55°C. However, the absence of standardized test methods for evaluating the electrical performance parameters of long-cycle solid-state batteries has hindered their widespread adoption. Existing standards for liquid lithium-ion batteries are inadequate due to differences in material properties and electrochemical behavior. Thus, we embarked on a comprehensive study to develop and validate test methods tailored to solid-state batteries, ensuring accurate assessment and fostering innovation in this field.
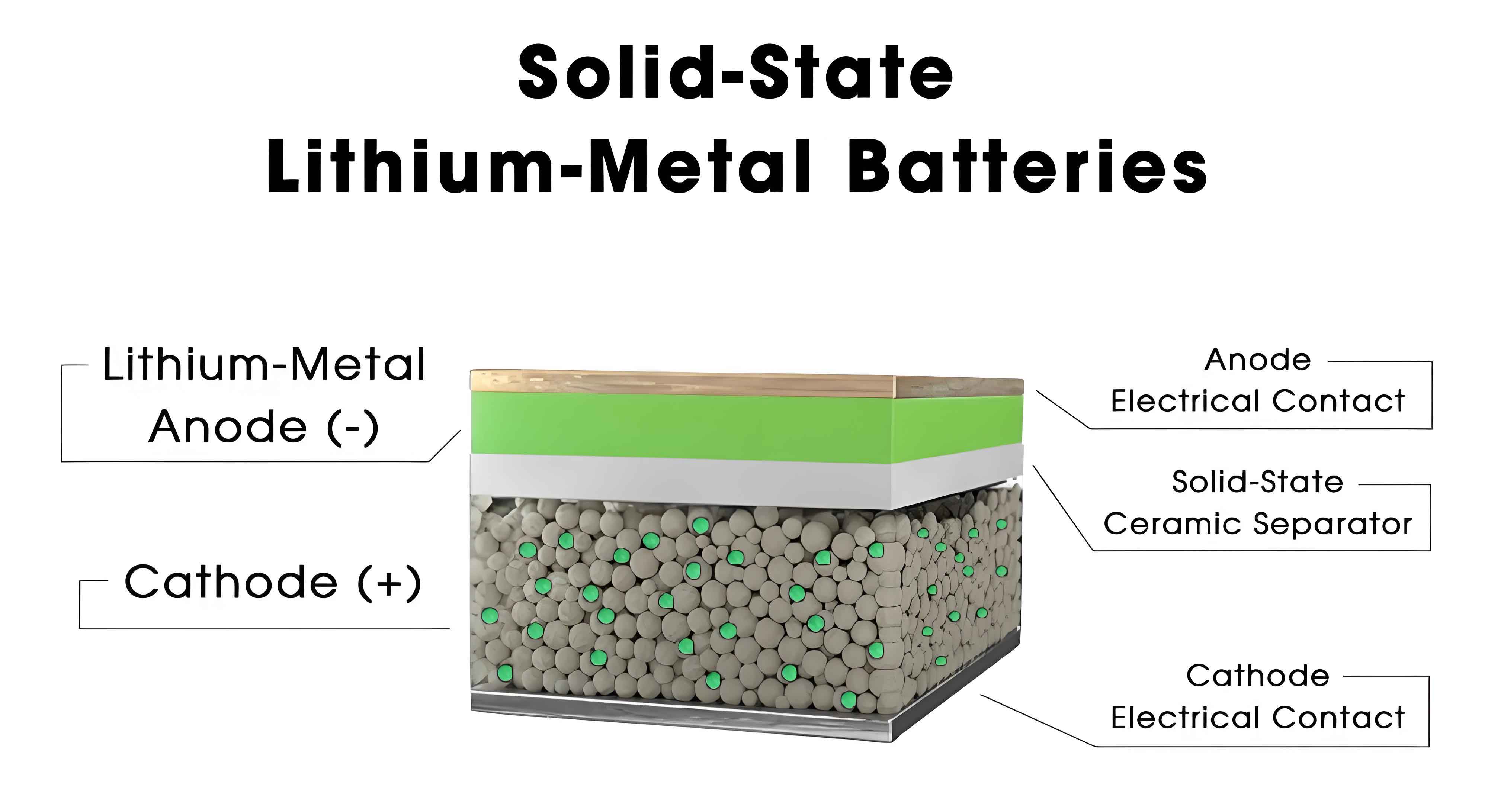
The core of our investigation involved analyzing key electrical performance parameters, such as gravimetric energy density, capacity retention under high-rate discharge, cycle life, low-temperature discharge capacity, and high-temperature discharge capacity. These parameters are critical for assessing the viability of solid-state batteries in real-world applications. For instance, the gravimetric energy density determines the battery’s weight efficiency, which is crucial for electric vehicles and aerospace applications. Similarly, cycle life and temperature resilience directly impact the longevity and safety of energy storage systems. Through experimental verification, we aimed to establish reliable test procedures that could be standardized for industry use.
To begin, we selected representative long-cycle solid-state battery samples for testing. These solid-state batteries were designed with advanced solid electrolytes, ensuring minimal risk of thermal incidents. The test equipment included an ET-1000L-C2 high-low temperature test chamber, a BTS-5V200A16CH battery tester, and a BT300 electronic balance, all calibrated to meet precision requirements. Our experiments were conducted under controlled environmental conditions, with temperatures maintained at 25°C ± 3°C, unless otherwise specified, to ensure consistency and reproducibility.
Analysis of Key Electrical Performance Parameters
Solid-state batteries demonstrate distinct advantages over liquid lithium-ion batteries in several key areas. For example, the gravimetric energy density of solid-state batteries can reach up to 350 Wh/kg, compared to approximately 250-300 Wh/kg for conventional batteries. This improvement is attributed to the higher ionic conductivity and stability of solid electrolytes. Additionally, solid-state batteries exhibit superior cycle life, with capacity retention exceeding 80% after 1,200 cycles, whereas liquid lithium-ion batteries typically degrade significantly after 800 cycles. The thermal stability of solid-state batteries is another standout feature; they maintain over 80% of their room-temperature discharge capacity at 80°C, while liquid batteries are prone to severe side reactions and failure at temperatures above 55°C.
To quantify these differences, we conducted a comparative analysis using the following table, which summarizes the performance metrics of solid-state batteries versus liquid lithium-ion batteries:
| Parameter | Solid-State Batteries | Liquid Lithium-Ion Batteries |
|---|---|---|
| Gravimetric Energy Density | ≥ 350 Wh/kg | 250-300 Wh/kg |
| Cycle Life (Capacity Retention) | ≥ 80% after 1,200 cycles | ~80% after 800 cycles |
| Low-Temperature Discharge (-20°C) | ~70% of room-temperature capacity | ~70% of room-temperature capacity |
| High-Temperature Discharge (80°C) | ~80% of room-temperature capacity | Degrades above 55°C |
| 2C Discharge Capacity Retention | ≥ 80% | Varies widely |
The data highlights the need for specialized test methods for solid-state batteries, as their performance characteristics deviate significantly from those of liquid-based systems. For instance, the solid electrolyte in solid-state batteries reduces the risk of dendrite formation, which is a common failure mechanism in liquid electrolytes, thereby enhancing cycle life. Moreover, the interfacial stability between the solid electrolyte and electrodes in solid-state batteries contributes to better high-rate performance and temperature tolerance.
Experimental Methodology for Electrical Performance Testing
Our experimental approach involved designing and executing tests for each key parameter, with a focus on reproducibility and accuracy. The test methods were developed based on industry standards but adapted to address the unique properties of solid-state batteries. Below, we detail the procedures, results, and analytical insights for each test, incorporating mathematical formulations where applicable.
Gravimetric Energy Density Test
The gravimetric energy density is a critical indicator of the energy storage efficiency per unit mass. For solid-state batteries, this parameter is essential for applications where weight is a constraint, such as in electric vehicles or portable devices. The test was conducted as follows: under room temperature conditions (25°C ± 3°C), the solid-state battery was charged at a constant current of 0.3C until the voltage reached 4.3V, followed by a constant voltage charge until the current dropped to 0.05C or below. After a 15-minute rest period, the battery was discharged at 0.3C to a cutoff voltage of 2.5V. The discharge energy (E) in watt-hours (Wh) was recorded, and the battery mass (m) in kilograms (kg) was measured using an electronic balance. The gravimetric energy density (E_w) was calculated using the formula:
$$E_w = \frac{E}{m}$$
In our tests, the discharge energy E was measured as 290.79 Wh, and the mass m was 0.83 kg, resulting in E_w = 350.34 Wh/kg. This value meets the requirement of E_w ≥ 350 Wh/kg for long-cycle solid-state batteries, demonstrating their superior energy density. The solid electrolyte in these solid-state batteries contributes to this high value by enabling higher operating voltages and reduced weight compared to liquid systems.
2C Discharge Capacity Retention Test
High-rate discharge capability is vital for applications requiring rapid power delivery, such as acceleration in electric vehicles. The 2C discharge capacity retention test evaluates the battery’s ability to maintain capacity under high current loads. The procedure began with charging the solid-state battery at 0.3C to 4.3V, followed by a constant voltage phase until the current reached 0.05C. After resting for 15 minutes, the battery was discharged at 0.3C to 2.5V to determine the initial capacity. Subsequently, it was recharged under the same conditions and discharged at 2C to 2.5V. The capacity retention ratio (R) was calculated as:
$$R = \frac{C_{2C}}{C_{0.3C}} \times 100\%$$
where C_{2C} is the discharge capacity at 2C, and C_{0.3C} is the initial capacity at 0.3C. Our measurements showed C_{0.3C} = 81.41 Ah and C_{2C} = 79.78 Ah, yielding R = 98.0%. This exceeds the minimum requirement of 80%, indicating that solid-state batteries can sustain high discharge rates without significant capacity loss. The robust interfacial contact in solid-state batteries minimizes polarization effects, which is crucial for high-rate performance.
Cycle Life Test
Cycle life is a key metric for assessing the longevity and durability of batteries. For long-cycle solid-state batteries, we aimed to verify capacity retention after 1,200 cycles. The test involved charging the battery at 0.3C to 4.2V, followed by a constant voltage charge until the current fell to 0.05C. After a 15-minute rest, the battery was discharged at 0.5C to 3.0V. This charge-discharge cycle was repeated 1,200 times. The capacity retention after N cycles is given by:
$$CR_N = \frac{C_N}{C_1} \times 100\%$$
where C_N is the discharge capacity at the Nth cycle, and C_1 is the initial capacity. In our experiment, C_1 was 70 Ah, and C_{1200} was 64 Ah, resulting in CR_{1200} = 91.4%. This surpasses the 80% threshold, underscoring the exceptional cycle stability of solid-state batteries. The solid electrolyte’s ability to suppress side reactions and mechanical degradation contributes to this prolonged cycle life.
Low-Temperature Discharge Capacity Test
Performance at low temperatures is critical for applications in cold climates. We evaluated the low-temperature discharge capacity by first charging the solid-state battery at 0.3C to 4.3V under room temperature, followed by a constant voltage charge to 0.05C. After resting, the battery was placed in a -20°C ± 2°C environment for 8 hours and then discharged at 1C to 2.5V. The low-temperature capacity retention (LTR) was calculated as:
$$LTR = \frac{C_{-20°C}}{C_{25°C}} \times 100\%$$
where C_{-20°C} is the discharge capacity at -20°C, and C_{25°C} is the capacity at 25°C. Our results showed C_{25°C} = 79.10 Ah and C_{-20°C} = 66.03 Ah, giving LTR = 83.47%. This meets the requirement of LTR ≥ 70%, demonstrating that solid-state batteries maintain reliable operation in frigid conditions. The solid electrolyte’s lower freezing point and reduced ionic resistance at low temperatures account for this performance.
High-Temperature Discharge Capacity Test
High-temperature performance is equally important, especially for applications exposed to heat. The test involved charging the solid-state battery at 0.3C to 4.3V under room temperature, followed by a constant voltage charge to 0.05C. After resting, the battery was stored at 80°C ± 2°C for 8 hours and discharged at 1C to 2.5V. The high-temperature capacity retention (HTR) is defined as:
$$HTR = \frac{C_{80°C}}{C_{25°C}} \times 100\%$$
With C_{25°C} = 79.53 Ah and C_{80°C} = 79.12 Ah, we obtained HTR = 99.48%, well above the 80% requirement. This highlights the thermal robustness of solid-state batteries, as the solid electrolyte prevents decomposition and gas formation that plague liquid electrolytes at high temperatures.
Development of Standardized Test Methods
Based on our experimental findings, we formulated a comprehensive set of test methods for evaluating the electrical performance of long-cycle solid-state batteries. These methods are designed to be practical, repeatable, and aligned with industry needs. The key parameters and their requirements are summarized in the table below:
| Test Parameter | Requirement | Test Condition |
|---|---|---|
| Gravimetric Energy Density | ≥ 350 Wh/kg | 25°C, 0.3C discharge to 2.5V |
| 2C Discharge Capacity Retention | ≥ 80% of initial capacity | 25°C, 2C discharge after 0.3C charge |
| Cycle Life | ≥ 80% capacity after 1,200 cycles | 25°C, 0.5C discharge to 3.0V |
| Low-Temperature Discharge Capacity | ≥ 70% of room-temperature capacity | -20°C, 1C discharge after 8h soak |
| High-Temperature Discharge Capacity | ≥ 80% of room-temperature capacity | 80°C, 1C discharge after 8h soak |
The test procedures are as follows:
- Charging Protocol: Under 25°C ± 3°C, discharge the solid-state battery at 0.3C to 2.5V, rest for 15 minutes, then charge at 0.3C to 4.4V, followed by a constant voltage charge until current ≤ 0.05C. Rest for 15 minutes. Note: C1 refers to the 1-hour discharge current in amperes, equivalent to the nominal capacity.
- Gravimetric Energy Density: After charging, discharge at 0.3C to 2.5V, record energy E (Wh), measure mass m (kg), and compute E_w = E / m.
- 2C Discharge Capacity Retention: After initial capacity measurement at 0.3C, recharge and discharge at 2C to 2.5V. Calculate retention ratio.
- Cycle Life: Charge at 0.3C to 4.25V, constant voltage to current ≤ 0.05C, rest 15 minutes, discharge at 0.5C to 2.8V. Repeat for 1,200 cycles.
- Low-Temperature Discharge Capacity: After initial capacity measurement, charge, rest, and soak at -20°C for 8 hours. Discharge at 1C to 2.5V.
- High-Temperature Discharge Capacity: After initial capacity measurement, charge, rest, and soak at 80°C for 8 hours. Discharge at 1C to 2.5V.
These methods have been integrated into internal product specifications for solid-state batteries, providing a foundation for quality control and performance validation. The use of solid-state batteries in these tests ensures that the methods are tailored to their unique characteristics, such as the absence of liquid electrolytes and enhanced interfacial stability.
Conclusion
Our research successfully addresses the gap in test methods for long-cycle solid-state batteries by developing and validating procedures for key electrical performance parameters. Through rigorous experimentation, we demonstrated that solid-state batteries achieve a gravimetric energy density of over 350 Wh/kg, maintain high capacity retention under extreme rates and temperatures, and exhibit exceptional cycle life exceeding 1,200 cycles. The proposed test methods, which include gravimetric energy density, 2C discharge capacity retention, cycle life, low-temperature discharge capacity, and high-temperature discharge capacity, provide a reliable framework for evaluating solid-state batteries in various applications.
The adoption of these methods will facilitate the standardization and commercialization of solid-state batteries, enabling manufacturers and users to assess performance consistently. Future work should focus on refining these tests for emerging solid-state battery technologies, such as those with sulfide or oxide-based electrolytes, and expanding them to include safety and abuse tolerance evaluations. As the demand for safer, higher-energy-density batteries grows, the insights from this study will contribute significantly to the advancement of solid-state battery technology, paving the way for more sustainable and efficient energy storage solutions.
