Sodium-ion batteries (SIBs) have emerged as a promising alternative to lithium-ion batteries (LIBs) due to the abundance and low cost of sodium resources, coupled with their similar working principles. As a key component, solid-state electrolytes significantly enhance the safety and cycling stability of SIBs by mitigating risks associated with liquid electrolytes, such as leakage and flammability. In this article, I review the fundamental working principles of SIBs and the recent advancements in solid-state electrolytes, focusing on polymer-based, inorganic, and gel polymer electrolytes. The chemical composition and structure of these electrolytes critically influence their ionic and electronic conductivity, which directly impacts the safety performance and cycle life of solid state batteries. Despite ongoing challenges, solid-state electrolytes offer immense potential for developing high-performance SIBs for applications like large-scale energy storage and electric vehicles.
The basic operation of SIBs involves the reversible migration of Na+ ions between the cathode and anode during charge and discharge cycles, analogous to LIBs. For instance, in a typical SIB with a NaMnO2 cathode and hard carbon anode, the discharge reactions can be represented as follows:
$$ \text{NaMnO}_2 \rightarrow \text{Na}_{(1-x)}\text{MnO}_2 + x\text{Na}^+ + x e^- \quad \text{(Cathode)} $$
$$ \text{C} + x\text{Na}^+ + x e^- \rightarrow \text{Na}_x\text{C} \quad \text{(Anode)} $$
Here, \( x \) denotes the number of Na+ ions involved. The electrolyte facilitates ion transport, and its properties—such as ionic conductivity and electrochemical stability—are paramount for battery efficiency. Solid-state electrolytes, including solid polymer electrolytes (SPE), inorganic solid electrolytes (ISE), and gel polymer electrolytes (GPE), have been extensively studied to overcome limitations of liquid systems. For example, the ionic conductivity (\( \sigma \)) of an electrolyte can be described by the Arrhenius equation:
$$ \sigma = \sigma_0 \exp\left(-\frac{E_a}{kT}\right) $$
where \( \sigma_0 \) is the pre-exponential factor, \( E_a \) is the activation energy for ion migration, \( k \) is Boltzmann’s constant, and \( T \) is the temperature. This relationship highlights the temperature dependence of conductivity, which is a critical factor in solid state battery performance.
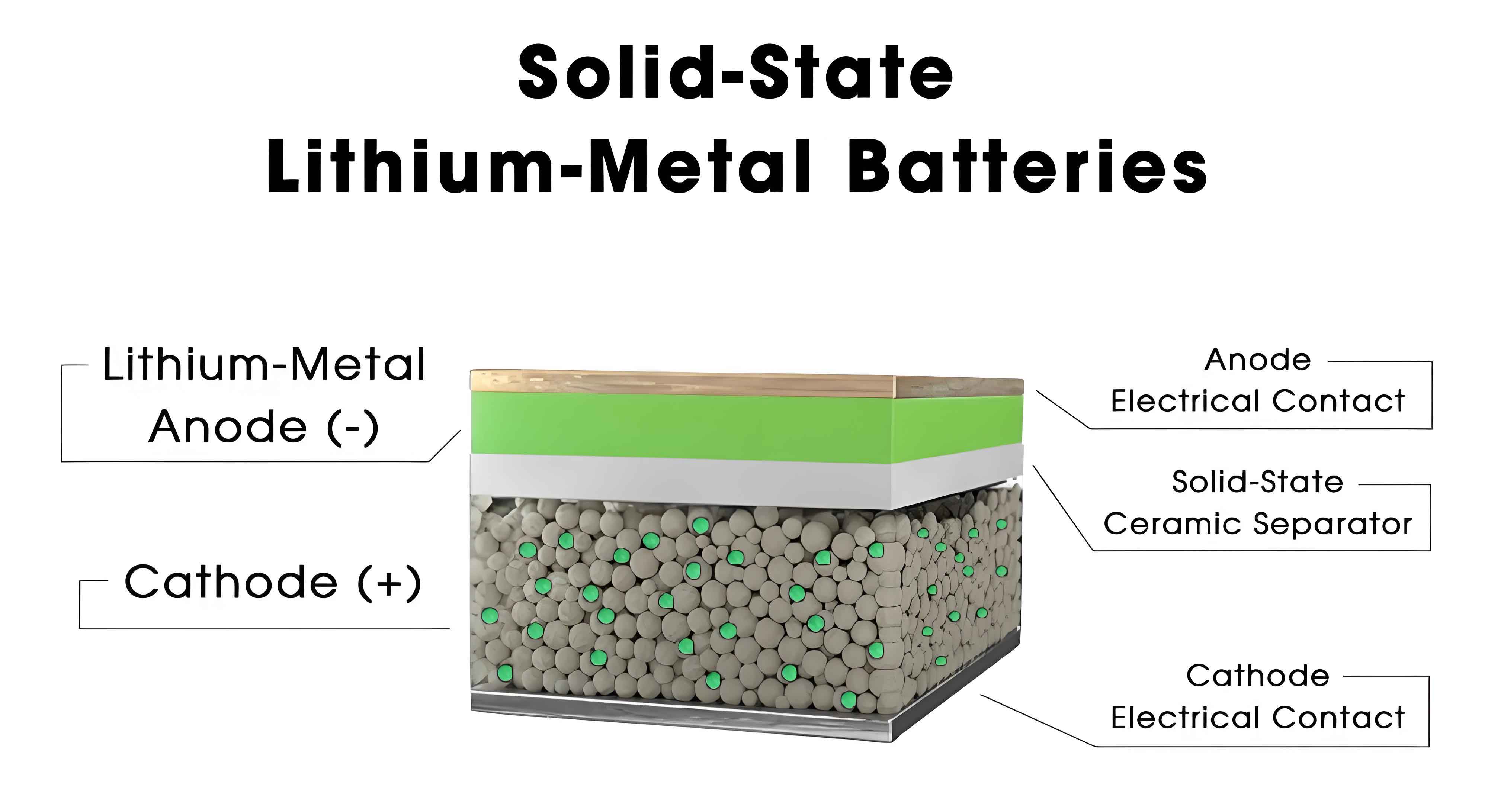
Solid polymer electrolytes (SPE) consist of a polymer matrix, such as poly(ethylene oxide) (PEO), blended with sodium salts like NaPF6 or NaTFSI. SPEs offer advantages like flexibility, ease of processing, and resistance to electrode volume changes. However, their room-temperature ionic conductivity is typically low (10−5 to 10−7 S/cm), necessitating elevated operating temperatures. For instance, PEO-based SPEs achieve conductivities of around 6.3 × 10−4 S/cm at 80°C, as demonstrated in studies on PEO-NaPF6 systems. The ion transference number (\( t_+ \)), which indicates the fraction of current carried by Na+ ions, is also a key parameter, often exceeding 0.5 in optimized SPEs. Other polymers like poly(vinyl alcohol) (PVA) and poly(acrylonitrile) (PAN) have been explored to enhance performance. Table 1 summarizes the properties of various SPEs.
| Polymer Matrix | Salt | Ionic Conductivity (S/cm) | Temperature (°C) | Transference Number (\( t_+ \)) |
|---|---|---|---|---|
| PEO | NaPF6 | 6.3 × 10−4 | 80 | 0.58 |
| PEO | NaFSI | 1 × 10−3 | 25 | — |
| PVDF | NaTFSI/NaDFOB | 3.1 × 10−4 | 23 | — |
| Cross-linked PEO | NaClO4/NaTFSI | 1 × 10−3 | 90 | — |
Inorganic solid electrolytes (ISE) exhibit high ionic conductivity and thermal stability, making them suitable for solid state batteries. Notable ISEs include β-Al2O3, NASICON-type, and sulfide-based electrolytes. β-Al2O3 electrolytes, such as Na-β′-Al2O3 and Na-β′′-Al2O3, feature layered structures with conductive planes for Na+ migration. The conductivity of Na-β′′-Al2O3 can reach 0.2–0.4 S/cm at 300°C, but their fabrication requires high sintering temperatures (1200–1500°C), limiting practicality. NASICON-type electrolytes, with the general formula Na1+xZr2SixP3−xO12 (0 ≤ x ≤ 3), offer 3D ion transport pathways. For x=2 (Na3Zr2Si2PO12), the room-temperature conductivity is 6.7 × 10−4 S/cm, driven by the open framework of SiO4 and PO4 tetrahedra. The ionic conductivity in NASICON can be modeled using the Nernst-Einstein relation:
$$ \sigma = \frac{n q^2 D}{kT} $$
where \( n \) is the carrier concentration, \( q \) is the charge, and \( D \) is the diffusion coefficient. Sulfide-based ISEs, like cubic Na3PS4, provide conductivities up to 2 × 10−4 S/cm at room temperature and exhibit low grain boundary resistance. Additionally, complex hydride-based electrolytes (e.g., NaBH4 derivatives) have shown promise, with conductivities of 4 mS/cm for Nido-NaB11H14. Table 2 compares key ISEs.
| Electrolyte Type | Composition | Ionic Conductivity (S/cm) | Temperature (°C) | Notes |
|---|---|---|---|---|
| β-Al2O3 | Na-β′′-Al2O3 | 0.2–0.4 | 300 | High-temperature operation |
| NASICON | Na3Zr2Si2PO12 | 6.7 × 10−4 | 25 | 3D diffusion network |
| Sulfide | Na3PS4 | 2.0 × 10−4 | 25 | Low grain boundary resistance |
| Complex Hydride | Nido-NaB11H14 | 4 × 10−3 | 20 | Wide electrochemical window |
Gel polymer electrolytes (GPE) combine the benefits of solid and liquid electrolytes by incorporating plasticizers (e.g., organic solvents or ionic liquids) into a polymer matrix. This results in improved ionic conductivity and interfacial contact while maintaining mechanical stability. PEO-based GPEs, such as those blended with Na3Zr2Si2PO12 (NZSP), achieve conductivities of 4.42 × 10−4 S/cm at room temperature and exhibit electrochemical stability up to 4.5 V vs. Na/Na+. Similarly, PAN-based GPEs with NaClO4 in EC/PC solvents show conductivities of 4.5 mS/cm at 25°C. PVDF-HFP-based GPEs, enhanced with Na3SbS3Se particles, demonstrate conductivities of 1.31 × 10−4 S/cm and critical current densities of 1.1 mA/cm², suppressing sodium dendrite growth. The ion transport in GPEs can be described by the Vogel-Tammann-Fulcher (VTF) equation:
$$ \sigma = A T^{-1/2} \exp\left(-\frac{B}{T – T_0}\right) $$
where \( A \) and \( B \) are constants, and \( T_0 \) is the ideal glass transition temperature. Other GPEs, like PVDF/PMMA blends, offer flexibility and high transference numbers (0.47), enabling stable cycling in flexible solid state batteries. Table 3 provides an overview of GPE performance.
| Polymer Matrix | Plasticizer/Additive | Ionic Conductivity (S/cm) | Temperature (°C) | Transference Number (\( t_+ \)) |
|---|---|---|---|---|
| PEO | NZSP/NaClO4 | 4.42 × 10−4 | 25 | — |
| PAN | EC/PC/NaClO4 | 4.5 × 10−3 | 25 | — |
| PVDF-HFP | Na3SbS3Se | 1.31 × 10−4 | 25 | — |
| PVDF-HFP | Ionic Liquid/NaTFSI | 1.9 × 10−3 | 30 | 0.27 |
| PVDF/PMMA | Gel Solvent | 5.07 × 10−4 | 25 | 0.47 |
Despite the progress, solid-state electrolytes face challenges such as low ionic conductivity at room temperature, poor interfacial stability with electrodes, and mechanical brittleness in inorganic systems. For instance, the interface between solid electrolytes and electrodes often leads to high resistance and dendrite formation, which can be mitigated by composite approaches. Future research should focus on developing hybrid materials, optimizing synthesis methods to reduce costs, and enhancing ion transport through nanostructuring. The integration of computational modeling and in-situ characterization techniques will accelerate the design of next-generation solid state batteries. Moreover, the scalability of solid-state SIBs for commercial applications requires addressing manufacturing hurdles and improving cycle life under practical conditions.
In conclusion, solid-state electrolytes are pivotal for advancing sodium-ion batteries, offering enhanced safety and longevity. Polymer electrolytes provide flexibility but need conductivity improvements, inorganic electrolytes excel in conductivity but require better mechanical properties, and gel electrolytes balance both yet face issues like solvent volatility. Continued innovation in material science and electrochemistry will unlock the full potential of solid state batteries, driving their adoption in renewable energy storage and portable electronics. As I reflect on the current state, it is clear that interdisciplinary efforts are essential to overcome existing barriers and realize efficient, cost-effective solid state batteries for a sustainable future.
