As the demand for high-energy-density and safe energy storage systems grows, all-solid-state batteries have emerged as a promising next-generation technology. Among various solid electrolyte materials, sulfide-based solid electrolytes stand out due to their exceptional ionic conductivity, which rivals or even surpasses that of liquid electrolytes. This makes sulfide-based all-solid-state batteries a focal point for research and industrial applications. Central to the development of these batteries is lithium sulfide (Li2S), a critical precursor for synthesizing high-performance sulfide solid electrolytes. In this article, we explore the fundamental properties, quality assessment, and scalable production methods of Li2S, emphasizing its pivotal role in advancing solid state battery technologies.
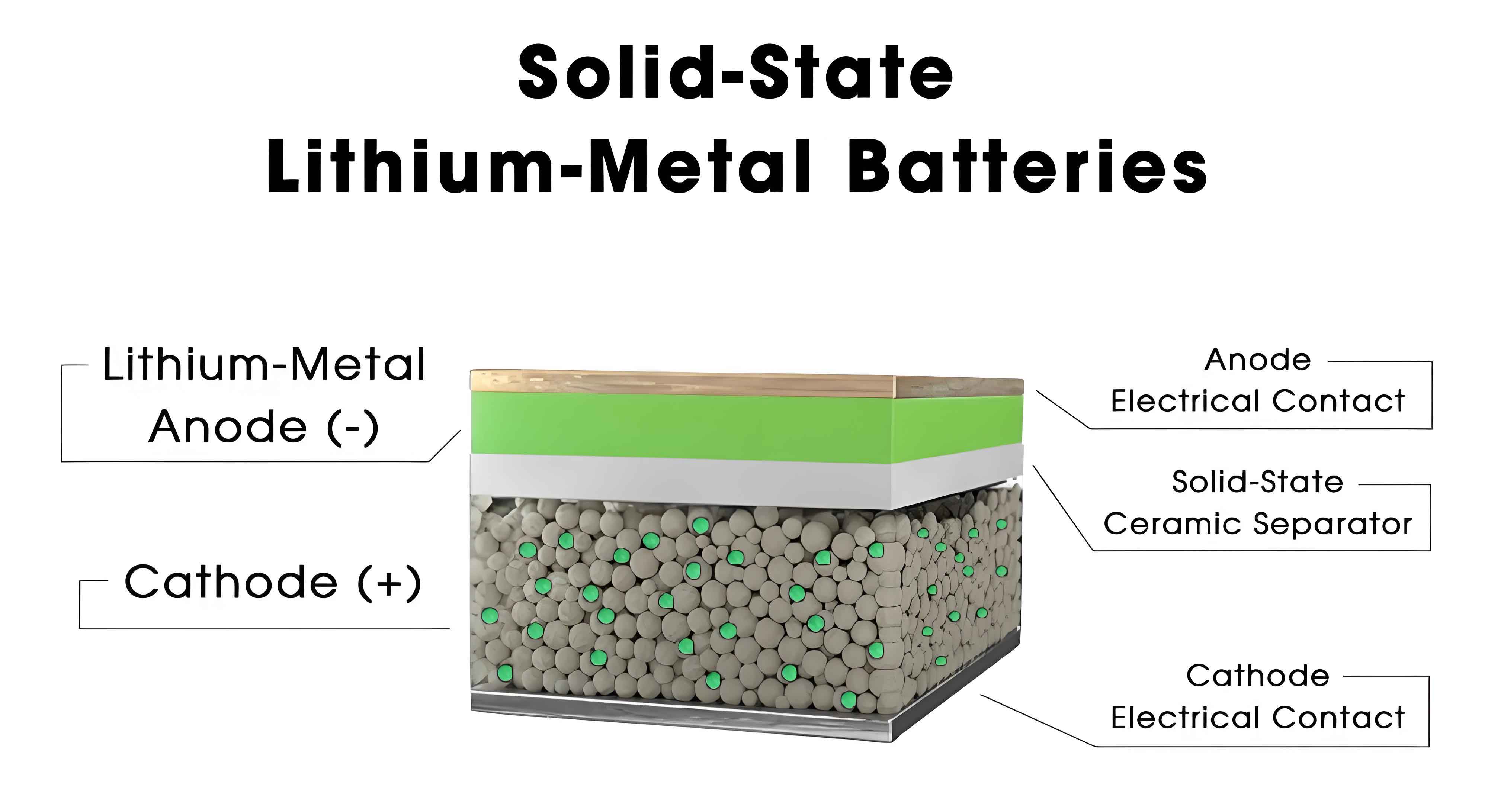
The significance of Li2S in solid state batteries cannot be overstated. It serves as the primary lithium and sulfur source in the synthesis of sulfide solid electrolytes, such as Li6PS5Cl and Li10GeP2S12, which exhibit room-temperature ionic conductivities exceeding 10 mS/cm. These electrolytes are essential for enabling all-solid-state batteries with enhanced safety, as they replace flammable liquid electrolytes, reducing risks of thermal runaway. Moreover, the economic viability of sulfide-based all-solid-state batteries heavily depends on the cost-effective production of high-purity Li2S. For instance, in the manufacturing of Li6PS5Cl electrolyte, Li2S constitutes approximately 43% of the mass and accounts for over 80% of the raw material cost. Thus, optimizing the synthesis and quality of Li2S is crucial for the widespread adoption of solid state batteries.
In this comprehensive review, we delve into the intrinsic properties of Li2S, including its crystal structure, reactivity, and stability. We then discuss the key quality parameters that influence the performance of sulfide solid electrolytes, such as purity, moisture content, and impurity levels. Furthermore, we evaluate various production routes for Li2S, ranging from high-activity methods to more sustainable approaches, and provide a comparative analysis based on safety, cost, and scalability. By addressing the current challenges and future directions, we aim to contribute to the development of efficient and economical Li2S production processes, ultimately accelerating the commercialization of sulfide-based all-solid-state batteries.
Fundamental Properties of Lithium Sulfide
Lithium sulfide (Li2S) is an inorganic compound with the chemical formula Li2S and a molar mass of 45.95 g/mol. It typically appears as a white crystalline powder but can exhibit grayish or yellowish tints due to impurities. The material has a melting point of 938°C and a density of 1.66 g/cm3. Li2S crystallizes in a cubic anti-fluorite structure, where sulfur anions form a cubic close-packed arrangement, and lithium cations occupy all tetrahedral interstitial sites. This structure is represented by the space group Fm3m, with a lattice parameter of approximately 5.71 Å.
The chemical reactivity of Li2S is a critical aspect for its handling and application in solid state batteries. It is highly hygroscopic and reacts vigorously with water, leading to hydrolysis reactions that produce lithium hydrogen sulfide (LiHS) and lithium hydroxide (LiOH), as shown in Equation (1). Further hydrolysis can release hydrogen sulfide (H2S) gas, which is toxic and poses safety hazards. Additionally, Li2S is susceptible to oxidation in air. At room temperature, it slowly oxidizes to form elemental sulfur and lithium oxide, as described in Equation (2). At elevated temperatures (above 300°C), it can be oxidized to lithium sulfate (Li2SO4), as indicated in Equation (3). These reactions underscore the need for strict control of moisture and oxygen during the production, storage, and processing of Li2S for all-solid-state batteries.
$$ \text{Li}_2\text{S} + \text{H}_2\text{O} \rightarrow \text{LiHS} + \text{LiOH} \quad (1) $$
$$ 2\text{Li}_2\text{S} + \text{O}_2 \rightarrow 2\text{Li}_2\text{O} + 2\text{S} \quad (2) $$
$$ \text{Li}_2\text{S} + 2\text{O}_2 \rightarrow \text{Li}_2\text{SO}_4 \quad (3) $$
The thermal stability of Li2S is another important property. It decomposes at high temperatures, releasing sulfur vapors, which can complicate high-temperature synthesis processes. The enthalpy of formation (ΔHf) for Li2S is approximately -447 kJ/mol, indicating its high stability. However, this also implies that the reduction of lithium sulfate or other precursors to form Li2S requires significant energy input, often through carbothermal or other reduction methods.
To summarize the key physical and chemical properties of Li2S, we present Table 1, which includes parameters relevant to its use in solid state batteries. Understanding these properties is essential for designing efficient synthesis routes and ensuring the material’s compatibility with sulfide solid electrolytes.
| Property | Value | Description |
|---|---|---|
| Chemical Formula | Li2S | Inorganic compound |
| Molar Mass | 45.95 g/mol | Molecular weight |
| Appearance | White powder | Can vary with impurities |
| Melting Point | 938°C | High melting point |
| Density | 1.66 g/cm3 | Cubic crystal structure |
| Crystal Structure | Cubic anti-fluorite | Space group Fm3m |
| Solubility | Soluble in polar solvents | e.g., water, ethanol |
| Hygroscopicity | High | Reacts with moisture |
| Oxidation Sensitivity | Moderate to high | Forms oxides and sulfates |
The ionic conductivity of Li2S itself is low, but when combined with other sulfides like P2S5, it forms highly conductive solid electrolytes for all-solid-state batteries. For example, the Li2S-P2S5 system can achieve ionic conductivities up to 10 mS/cm after appropriate processing. The performance of these electrolytes is highly dependent on the purity and particle size of Li2S, as impurities can lead to secondary phases that hinder ion transport. Thus, controlling the properties of Li2S is paramount for optimizing solid state battery performance.
Quality Assessment of Lithium Sulfide
The quality of Li2S directly impacts the electrochemical performance of sulfide solid electrolytes in all-solid-state batteries. Key evaluation parameters include whiteness, phase purity, moisture content, solvent residues, and carbon content. Each of these factors can introduce defects or impurities that degrade ionic conductivity, promote electronic conduction, or cause instability in solid state batteries.
Whiteness and Color: Pure Li2S is white, but commercial samples often exhibit colors ranging from gray to yellow due to impurities such as polysulfides (Li2Sx, x>2) or transition metal contaminants. These colored impurities typically arise from incomplete reactions or contamination during synthesis. For instance, polysulfides form when there is an excess of sulfur or non-uniform reaction conditions. The presence of polysulfides can be detected using X-ray photoelectron spectroscopy (XPS), where the S2p peak at around 161.8 eV corresponds to polysulfide species. Whiteness is often assessed visually or with a whiteness meter, and it serves as a preliminary indicator of purity. In solid state batteries, polysulfide impurities can lead to increased electronic conductivity and promote decomposition reactions, reducing the lifespan of all-solid-state batteries.
Phase Purity: The phase composition of Li2S is critical for maintaining stoichiometric accuracy in sulfide solid electrolytes. Common impurity phases include lithium oxide (Li2O), lithium hydroxide (LiOH), and lithium carbonate (Li2CO3), which form due to exposure to moisture or carbon dioxide during processing or storage. These impurities can react with electrolyte precursors like P2S5, leading to non-conductive phases such as Li3PO4 and altering the electrolyte’s composition. X-ray diffraction (XRD) is commonly used to identify these phases. For example, diffraction peaks at 2θ values of 27.1°, 31.5°, and 45.2° correspond to Li2S, while peaks at 32.1° and 34.8° may indicate LiOH or Li2CO3. High phase purity ensures that the synthesized solid electrolytes exhibit optimal ionic conductivity for all-solid-state batteries.
Moisture Content: Li2S is highly hygroscopic, and even trace amounts of moisture can trigger hydrolysis during storage, generating LiOH and H2S. This not only reduces purity but also poses safety risks. The moisture content is typically measured using Karl Fischer titration, and levels below 100 mg/kg are desirable for stable storage. Experimental studies show that Li2S with moisture content above 2000 mg/kg undergoes significant degradation within days, forming LiOH phases as confirmed by XRD. Controlling moisture is essential for the long-term stability of Li2S and the reproducibility of solid electrolyte synthesis in all-solid-state batteries.
Solvent Residues: In wet chemical synthesis routes, solvents like ethanol or N-methylpyrrolidone (NMP) are used for purification or reaction media. However, these solvents can form complexes with Li2S and remain trapped in the product. Upon heating, residual solvents may decompose into carbonaceous species, increasing the electronic conductivity of solid electrolytes. This can lead to internal short circuits in all-solid-state batteries. Thermogravimetric analysis (TGA) coupled with gas chromatography-mass spectrometry (GC-MS) is employed to quantify solvent residues. For instance, Li2S synthesized via ethanol-based methods may retain up to 40% solvent after drying at 100°C, requiring temperatures above 300°C for complete removal.
Carbon Content: Carbon impurities often originate from organic precursors or reduction agents used in synthesis, such as in carbothermal reduction. These impurities elevate the electronic conductivity of solid electrolytes, facilitating lithium dendrite growth and electrolyte decomposition in all-solid-state batteries. Carbon content is measured using techniques like Raman spectroscopy, infrared carbon-sulfur analysis, or by assessing the electronic conductivity of the material. Keeping carbon levels low is crucial for maintaining the insulating nature of solid electrolytes and ensuring the safety of solid state batteries.
Table 2 summarizes the key quality parameters for Li2S, along with their measurement methods and implications for solid state battery performance. Establishing standardized quality control protocols is vital for the industrial production of high-purity Li2S tailored to all-solid-state batteries.
| Parameter | Measurement Method | Target Value | Impact on Solid State Batteries |
|---|---|---|---|
| Whiteness | Visual/Whiteness meter | High whiteness | Indicates low polysulfide and metal impurities |
| Phase Purity | X-ray diffraction (XRD) | Single-phase Li2S | Prevents non-conductive phases in electrolytes |
| Moisture Content | Karl Fischer titration | < 100 mg/kg | Ensures storage stability and prevents hydrolysis |
| Solvent Residues | TGA-GC-MS | Minimal residues | Avoids carbonization and high electronic conductivity |
| Carbon Content | Raman/IR analysis | Low carbon levels | Reduces risk of dendrites and short circuits |
Overall, rigorous quality assessment of Li2S is indispensable for producing reliable sulfide solid electrolytes. As the development of all-solid-state batteries progresses, continuous improvement in these parameters will enhance the performance and safety of solid state batteries.
Synthesis Routes for Lithium Sulfide
The production of Li2S involves various synthesis routes, each with distinct advantages and challenges. These methods can be broadly categorized into high-activity processes using reactive lithium or sulfur sources, and more stable processes employing economical precursors. The choice of synthesis route affects the cost, safety, and quality of Li2S, which in turn influences its applicability in all-solid-state batteries.
High-Activity Lithium/Sulfur Source Processes
These processes utilize highly reactive precursors, such as metallic lithium or hydrogen sulfide, to achieve high-purity Li2S with excellent yield. However, they often involve safety risks and high costs.
Lithium-Sulfur Direct Combination: This method involves the direct reaction between metallic lithium and sulfur powder, as shown in Equation (4). The reaction is highly exothermic, with a Gibbs free energy change (ΔG) of approximately -439 kJ/mol, leading to self-propagating high-temperature synthesis (SHS). The product is typically high-purity Li2S with whiteness exceeding 83% and purity above 99.9%. However, the process requires inert atmosphere conditions to prevent oxidation and poses risks of thermal runaway due to intense heat release. Scaling up this method necessitates advanced cooling systems and controlled feeding mechanisms to manage the reaction kinetics. Despite these challenges, it is widely used for small-scale production of Li2S for research and development in solid state batteries.
$$ 2\text{Li} + \text{S} \rightarrow \text{Li}_2\text{S} \quad (4) $$
Hydrogen Sulfide Neutralization: In this route, H2S gas reacts with lithium hydroxide or other lithium salts to form Li2S, as described in Equation (5). This process is efficient and can utilize H2S from industrial waste streams, adding economic value. However, H2S is toxic and flammable, requiring strict safety measures. Additionally, side reactions, such as the catalytic decomposition of H2S on Li2S surfaces, can produce polysulfides and hydrogen gas, as shown in Equations (6) and (7). Purification of H2S is essential to minimize impurities. This method is particularly suitable for modular production near H2S sources, reducing transportation hazards and supporting the sustainable manufacturing of materials for all-solid-state batteries.
$$ \text{H}_2\text{S} + 2\text{LiOH} \rightarrow \text{Li}_2\text{S} + 2\text{H}_2\text{O} \quad (5) $$
$$ \text{H}_2\text{S} + \text{Li}_2\text{S} \rightarrow \text{Li}_2\text{S}_2 + \text{H}_2 \quad (6) $$
$$ \text{H}_2\text{S}_x + 2\text{LiOH} \rightarrow \text{Li}_2\text{S}_x + 2\text{H}_2\text{O} \quad (7) $$
Chemically Stable Lithium/Sulfur Source Processes
These approaches use less reactive and more affordable precursors, offering improved safety and scalability for industrial production of Li2S. They include reduction methods and metathesis reactions.
Hydrazine Hydrate Reduction: This method employs hydrazine hydrate (N2H4·H2O) as a reducing agent to convert sulfur and lithium hydroxide into Li2S in an alkaline medium, as per Equation (8). The reaction proceeds at room temperature and is followed by heat treatment at 400–600°C to complete the reduction. It yields Li2S with purity up to 99.95%. The key advantages are low cost and mild reaction conditions. However, hydrazine is toxic and regulated, necessitating closed-system operations. Impurities such as polysulfides and moisture must be controlled to ensure the quality of Li2S for use in solid state batteries.
$$ 2\text{S} + 4\text{LiOH} + \text{N}_2\text{H}_4 \rightarrow 2\text{Li}_2\text{S} + \text{N}_2 + 4\text{H}_2\text{O} \quad (8) $$
Liquid-Phase Metathesis: This process involves a double displacement reaction between sodium sulfide (Na2S) and lithium salts, such as lithium chloride (LiCl), in a solvent like ethanol, as shown in Equation (9). The solubility differences drive the reaction forward, with Li2S precipitating out. Anti-solvents can be added to enhance precipitation, and solvents are recycled via distillation. The main benefits include low material costs and potential for continuous production. However, solvent residues and impurities from Na2S can affect product purity, requiring additional purification steps. This method is promising for large-scale synthesis of Li2S for all-solid-state batteries, provided that solvent management is optimized.
$$ \text{Na}_2\text{S} + 2\text{LiCl} \rightarrow \text{Li}_2\text{S} + 2\text{NaCl} \quad (9) $$
Thermal Reduction Routes: Carbothermal reduction is a prominent example, where lithium sulfate (Li2SO4) is reduced by carbon at high temperatures, as per Equation (10). This method uses inexpensive raw materials and is environmentally benign, as the precursors are stable in air. The reaction typically occurs above 635°C and can achieve purities over 99.5% with optimized conditions. However, it consumes significant energy and has low yield due to the release of carbon dioxide. Impurities like carbon residues and lithium carbonates may form, necessitating post-synthesis purification. Recent advances involve using organic carbon sources to improve reactivity and purity, making this route viable for mass production of Li2S for solid state batteries.
$$ \text{Li}_2\text{SO}_4 + 2\text{C} \rightarrow \text{Li}_2\text{S} + 2\text{CO}_2 \quad (10) $$
Other thermal methods include aluminothermal or magnesothermal reduction, where metals like aluminum or magnesium serve as reducing agents. These processes can be efficient but require separation of metal oxide by-products, adding complexity.
Table 3 compares the key synthesis routes for Li2S, highlighting their reaction conditions, advantages, and limitations in the context of all-solid-state battery applications.
| Synthesis Method | Reaction Equation | Temperature | Advantages | Disadvantages |
|---|---|---|---|---|
| Lithium-Sulfur Combination | 2Li + S → Li2S | Room temp to high | High purity, simple | Safety risks, cost |
| H2S Neutralization | H2S + 2LiOH → Li2S + 2H2O | Low to moderate | Waste utilization | Toxicity, side reactions |
| Hydrazine Reduction | 2S + 4LiOH + N2H4 → 2Li2S + N2 + 4H2O | Room temp to 600°C | Low cost, mild conditions | Toxicity, impurity control |
| Liquid-Phase Metathesis | Na2S + 2LiCl → Li2S + 2NaCl | Room temp | Scalable, continuous | Solvent residues, purification needed |
| Carbothermal Reduction | Li2SO4 + 2C → Li2S + 2CO2 | >635°C | Low cost, stable precursors | High energy, low yield |
In summary, the selection of a synthesis route for Li2S depends on factors such as purity requirements, safety considerations, and economic feasibility. For the advancement of all-solid-state batteries, developing cost-effective and scalable methods is essential.
Comparative Analysis of Synthesis Technologies
To evaluate the industrial feasibility of different Li2S synthesis routes, we analyze them based on product quality, safety, economic factors, and scalability. This analysis helps identify the most promising methods for supporting the growth of solid state batteries.
Product Quality Analysis: High-activity processes, such as lithium-sulfur direct combination and H2S neutralization, typically yield Li2S with superior purity (≥99.9%) and minimal impurities. These methods are ideal for producing high-grade material for research and premium solid state batteries. In contrast, stable precursor routes, like carbothermal reduction and liquid-phase metathesis, often require additional purification steps to achieve comparable purity. For instance, carbothermal-derived Li2S may contain carbon residues or lithium carbonates, which can be removed via solvent extraction or heat treatment. Liquid-phase methods face challenges with solvent and moisture retention, leading to potential degradation during storage. Therefore, while high-activity routes excel in quality, they may not be suitable for large-scale production due to safety and cost constraints.
Safety Considerations: Safety is a critical factor in scaling up Li2S production. High-activity methods involve significant risks: metallic lithium is pyrophoric, H2S is toxic, and hydrazine is hazardous. These require specialized equipment and strict protocols to prevent accidents. Stable precursor routes are generally safer; for example, carbothermal reduction uses non-hazardous materials, and liquid-phase metathesis operates under mild conditions. However, solvent handling in liquid-phase methods introduces flammability concerns, and high-temperature processes demand robust thermal management. A risk assessment matrix can prioritize methods based on hazard levels, with stable routes being more favorable for mass production of Li2S for all-solid-state batteries.
Economic Evaluation: The cost of Li2S production is dominated by raw materials and energy consumption. Using the formula for cost per ton of Li2S, we can estimate the economic viability of each method. For example, in carbothermal reduction, the cost is primarily from lithium sulfate and carbon, resulting in a raw material cost of approximately $13,610 per ton of Li2S. In contrast, lithium-sulfur combination uses expensive metallic lithium, leading to costs above $21,260 per ton. H2S neutralization and hydrazine reduction have intermediate costs, around $14,000–$15,000 per ton, depending on lithium source prices. Liquid-phase metathesis may incur additional costs for solvent recycling and purification. Energy costs also play a role; high-temperature processes like carbothermal reduction consume more energy, whereas room-temperature methods are more efficient. Overall, carbothermal and hydrazine routes offer the best balance of cost and safety for industrial-scale production of Li2S for solid state batteries.
Table 4 provides a detailed comparison of the synthesis technologies, including key metrics such as raw material cost, safety risk, and scalability. This table aids in decision-making for manufacturers aiming to produce Li2S for all-solid-state batteries.
| Technology | Raw Material Cost ($/ton Li2S) | Safety Risk | Scalability | Product Purity | Environmental Impact |
|---|---|---|---|---|---|
| Lithium-Sulfur Combination | >21,260 | High | Moderate | Very High | Low (if controlled) |
| H2S Neutralization | ~14,610 | High | High (modular) | High | Medium (waste utilization) |
| Hydrazine Reduction | ~14,750 | Medium | High | High | Medium (toxicity) |
| Liquid-Phase Metathesis | ~15,010 | Low-Medium | High | Medium-High | Medium (solvent use) |
| Carbothermal Reduction | ~13,610 | Low | High | Medium-High | Low (CO2 emission) |
Future Directions for Scalable Production: To meet the growing demand for Li2S in all-solid-state batteries, several strategies can be employed. For high-activity routes, improving safety through process intensification and advanced reactor design is crucial. For example, using lithium nitride additives in lithium-sulfur reactions can enhance heat dissipation and reduce risks. For stable precursor routes, optimizing reaction conditions to minimize impurities and energy consumption is key. Carbothermal reduction can be integrated with lithium extraction from ores or recycled batteries, leveraging low-cost sulfate sources. H2S neutralization can be deployed near natural gas processing plants to utilize waste H2S sustainably. Additionally, developing continuous-flow systems for liquid-phase metathesis could increase throughput and reduce costs. By focusing on these areas, the production of Li2S can become more efficient and economical, supporting the widespread adoption of sulfide-based all-solid-state batteries.
In conclusion, the choice of synthesis technology for Li2S involves trade-offs between quality, safety, and cost. For the solid state battery industry, prioritizing scalable and safe methods like carbothermal reduction or hydrazine reduction will be essential for achieving cost-effective production of high-performance solid electrolytes.
Conclusion
Lithium sulfide is undeniably a cornerstone material for the development of sulfide-based all-solid-state batteries. Its role as a primary precursor for high-ionic-conductivity solid electrolytes underscores its importance in achieving safe, high-energy-density energy storage systems. Throughout this article, we have examined the fundamental properties of Li2S, emphasizing its reactivity and stability, which necessitate careful handling and quality control. We have also detailed the critical quality parameters—such as whiteness, phase purity, and moisture content—that directly influence the performance of solid electrolytes in all-solid-state batteries. Furthermore, we have explored various synthesis routes, from high-activity methods to more sustainable approaches, and provided a comparative analysis based on product quality, safety, and economics.
The future of Li2S production lies in overcoming the current challenges of impurity control, safety risks, and high costs. Innovations in process optimization, such as enhancing carbothermal reduction with organic carbon sources or deploying H2S neutralization in modular units, hold great promise for scalable and cost-effective manufacturing. Additionally, integrating Li2S synthesis with lithium recovery from sustainable sources can further reduce environmental impact and costs. As the demand for all-solid-state batteries continues to rise, driven by applications in electric vehicles and grid storage, the advancement of Li2S production technologies will be pivotal. By fostering collaboration between academia and industry, we can accelerate the commercialization of high-quality Li2S and, consequently, high-performance solid state batteries, paving the way for a safer and more efficient energy future.
In summary, the journey toward efficient all-solid-state batteries relies heavily on the optimization of Li2S. Through continuous research and development, we can unlock the full potential of this material, enabling the next generation of solid state batteries to meet the evolving needs of society.
