As I observe the rapid evolution of energy storage technologies, I am increasingly convinced that solid state batteries represent a transformative leap forward. Unlike conventional lithium-ion batteries, which rely on liquid electrolytes, solid state batteries utilize solid electrodes and solid electrolytes, offering a host of advantages that could redefine industries from electric vehicles to grid storage. In this analysis, I will delve into the intricacies of solid state batteries, exploring their benefits, technical pathways, current industry landscape, challenges, and the profound impact they are poised to have on existing lithium battery ecosystems. Throughout this discussion, I will emphasize the recurring themes of innovation and disruption associated with solid state batteries, supported by data, tables, and formulas to provide a comprehensive perspective.
To begin, let me clarify what sets solid state batteries apart. The core innovation lies in the replacement of flammable liquid electrolytes with solid alternatives, which can be based on oxides, sulfides, or polymers. This fundamental change imparts superior safety, as solid state batteries are less prone to thermal runaway and can operate reliably under extreme conditions such as short circuits, high temperatures, or physical impacts. Moreover, solid state batteries exhibit higher energy densities, often quantified by the formula for gravimetric energy density: $$ E_g = \frac{Q \times V}{m} $$ where \( E_g \) is the energy density in Wh/kg, \( Q \) is the charge capacity in Ah, \( V \) is the voltage, and \( m \) is the mass. For instance, theoretical values for solid state batteries can exceed 500 Wh/kg, compared to around 250-300 Wh/kg for liquid lithium-ion counterparts. Additionally, their solid nature allows for miniaturization and enhanced cycle life, making them ideal for applications where space and durability are critical.
The advantages of solid state batteries are not merely theoretical; they translate into tangible benefits across multiple domains. In electric vehicles, for example, the higher energy density means longer driving ranges without increasing battery size, while the improved safety reduces risks of fires. To illustrate, consider the following table summarizing key performance metrics compared to traditional batteries:
| Parameter | Liquid Lithium-ion Batteries | Solid State Batteries |
|---|---|---|
| Energy Density (Wh/kg) | ~250 | ~400-500 (projected) |
| Safety (Thermal Runaway Risk) | High | Low |
| Cycle Life (Cycles) | 500-1000 | 1000+ |
| Operating Temperature Range (°C) | -20 to 60 | -40 to 100 |
As I reflect on these benefits, it becomes clear why solid state batteries are attracting such intense interest. The shift toward solid state batteries is not just incremental; it is a paradigm shift that could address many limitations of current energy storage systems. For instance, the solid electrolyte interface reduces side reactions, leading to longer lifespan, as modeled by the Arrhenius equation for degradation: $$ k = A e^{-\frac{E_a}{RT}} $$ where \( k \) is the rate constant, \( A \) is the pre-exponential factor, \( E_a \) is the activation energy, \( R \) is the gas constant, and \( T \) is temperature. In solid state batteries, higher \( E_a \) values for degradation pathways contribute to enhanced stability.
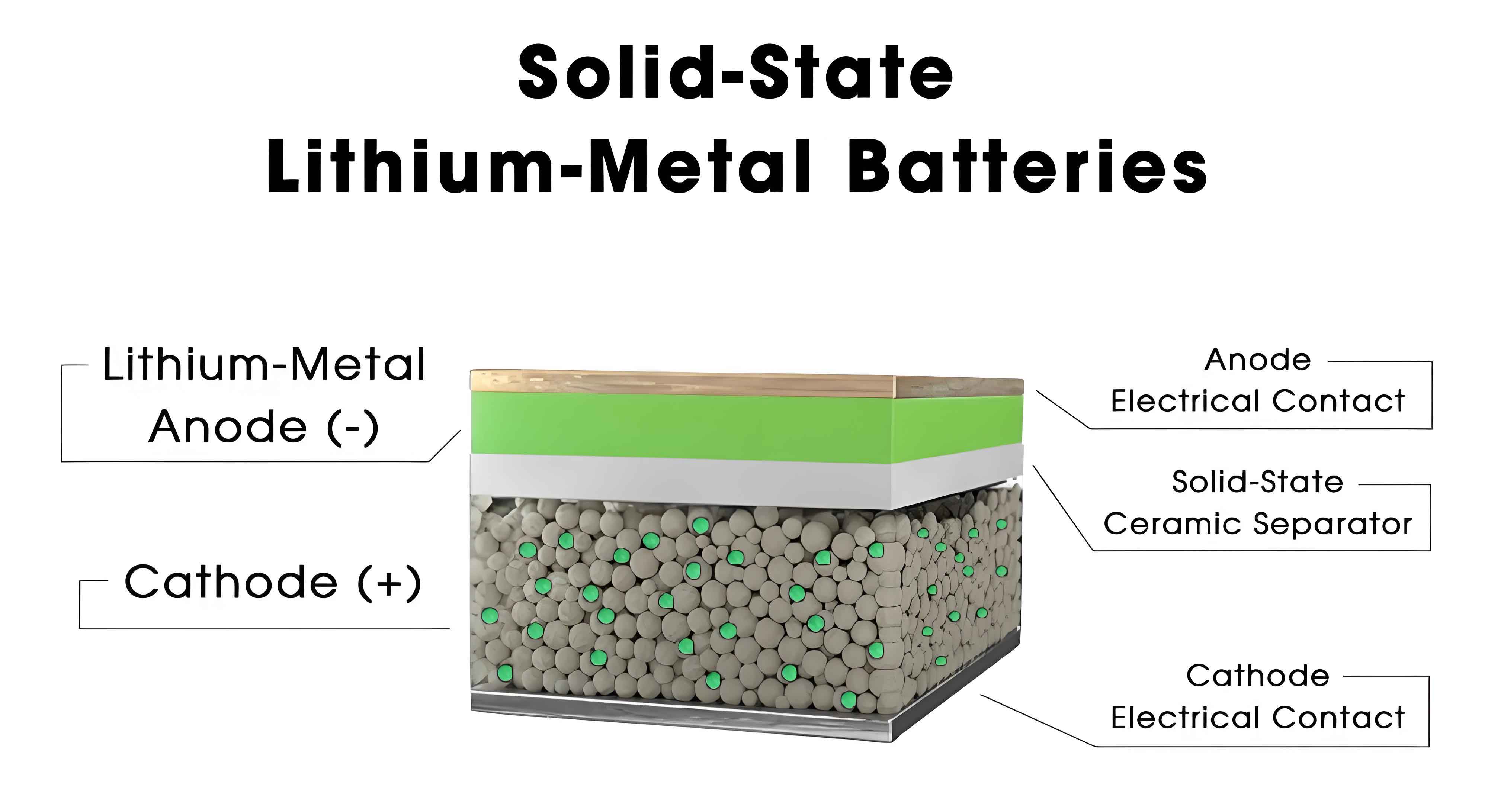
Moving to the technical landscape, I see a diverse array of pathways for solid state batteries, primarily categorized into semi-solid and all-solid configurations, with further subdivisions based on electrolyte materials. Oxide-based and sulfide-based electrolytes are currently the most prominent, owing to their balance of performance and manufacturability. Oxide electrolytes, for example, offer good ionic conductivity and stability, but may require high-temperature processing. Sulfide electrolytes, while challenging to synthesize due to sensitivity to moisture, provide excellent ionic conductivity, often described by the Nernst-Einstein relation: $$ \sigma = \frac{n q^2 D}{k_B T} $$ where \( \sigma \) is the ionic conductivity, \( n \) is the charge carrier density, \( q \) is the charge, \( D \) is the diffusion coefficient, \( k_B \) is Boltzmann’s constant, and \( T \) is temperature. Polymer-based solid state batteries, though less common, offer flexibility and ease of fabrication, but typically have lower conductivity. The following table outlines the key characteristics of these electrolyte types:
| Electrolyte Type | Ionic Conductivity (S/cm) | Stability | Manufacturing Complexity |
|---|---|---|---|
| Oxide | 10^{-4} to 10^{-3} | High | Moderate |
| Sulfide | 10^{-3} to 10^{-2} | Low (moisture-sensitive) | High |
| Polymer | 10^{-5} to 10^{-4} | Moderate | Low |
In my assessment, the industry is currently in a phase of vibrant competition, with numerous players advancing both semi-solid and all-solid state batteries. Semi-solid variants, which incorporate some liquid components, are already seeing commercialization, serving as a stepping stone to full solid state batteries. All-solid versions, while still in development, promise the ultimate in performance and safety. I have noted that leading companies are investing heavily in research, with some achieving milestones in pilot production. This progress is driven by collaborative efforts across academia, industry, and government, fostering an ecosystem where innovations in solid state batteries can flourish. For example, the development of thin-film solid state batteries leverages advancements in materials science, enabling applications in wearable electronics and IoT devices.
However, as I delve deeper, I recognize that the path to widespread adoption of solid state batteries is fraught with challenges. First and foremost are the technical hurdles. The solid-solid interface between electrodes and electrolytes in solid state batteries often leads to poor contact, increasing interfacial resistance and impairing performance. This can be modeled using the equation for charge transfer resistance: $$ R_{ct} = \frac{RT}{nF j_0} $$ where \( R_{ct} \) is the charge transfer resistance, \( n \) is the number of electrons, \( F \) is Faraday’s constant, and \( j_0 \) is the exchange current density. Additionally, the use of lithium metal anodes in solid state batteries introduces risks of dendrite formation, which can cause short circuits. Dendrite growth follows a relationship like: $$ L_d = k t^{1/2} $$ where \( L_d \) is the dendrite length, \( k \) is a constant, and \( t \) is time. Mitigating these issues requires novel materials and engineering solutions, such as composite electrolytes or protective coatings.
Another significant barrier is the high cost of manufacturing solid state batteries. The materials involved, such as pre-lithiated silicon-carbon anodes, high-nickel cathodes, and solid electrolytes, are substantially more expensive than those used in liquid lithium-ion batteries. For instance, the cost of silicon-based anodes can be three times that of graphite, as shown in the table below:
| Component | Traditional Lithium-ion Cost ($/kWh) | Solid State Battery Cost ($/kWh, estimated) |
|---|---|---|
| Anode (Graphite) | 50-100 | 150-300 (for silicon/lithium metal) |
| Cathode (NMC) | 100-150 | 200-250 (for high-nickel variants) |
| Electrolyte | 20-50 | 100-200 (for solid electrolytes) |
Moreover, the production processes for solid state batteries are less mature, requiring specialized equipment and controlled environments. This elevates capital expenditures and slows down scaling. In my view, cost reduction will hinge on economies of scale and technological breakthroughs, such as dry processing methods that eliminate solvents. The total cost of ownership for solid state batteries can be analyzed using life-cycle cost models: $$ C_{total} = C_{cap} + \sum_{t=1}^{n} \frac{C_{op}(t) + C_{main}(t)}{(1+r)^t} $$ where \( C_{cap} \) is the capital cost, \( C_{op} \) is operating cost, \( C_{main} \) is maintenance cost, \( r \) is the discount rate, and \( n \) is the lifespan. Currently, the high upfront cost of solid state batteries makes them less competitive, but as production ramps up, I anticipate significant declines.
Fast charging efficiency is another area where solid state batteries face limitations. The ionic conductivity of solid electrolytes is typically an order of magnitude lower than that of liquid electrolytes, leading to slower charge times. This can be expressed by the formula for charging time: $$ t_{charge} = \frac{Q}{I} \times \frac{1}{\eta} $$ where \( t_{charge} \) is the charging time, \( Q \) is the capacity, \( I \) is the current, and \( \eta \) is the efficiency. For electric vehicles, this translates to longer wait times, which could hinder adoption if not addressed. Research is focused on improving conductivity through doping or nanostructuring, but practical solutions remain elusive. In the interim, hybrid approaches that combine solid and liquid elements may offer a compromise.
Supply chain considerations also weigh heavily on my mind. The transition to solid state batteries necessitates a complete overhaul of existing supply chains, which are optimized for liquid lithium-ion systems. Key raw materials, such as zirconium compounds for oxide electrolytes, are subject to geopolitical risks and price volatility. For example, zirconia (ZrO₂) and related compounds are largely imported, creating dependencies that could disrupt production. The supply-demand dynamics can be modeled using simple economic formulas: $$ P = a – bQ $$ where \( P \) is price, \( Q \) is quantity, and \( a \) and \( b \) are constants. To build resilience, I advocate for diversifying sources and investing in domestic production capabilities. Additionally, recycling infrastructure for solid state batteries is underdeveloped, posing environmental challenges. The efficiency of recycling can be quantified by: $$ \eta_{recycle} = \frac{M_{recovered}}{M_{input}} \times 100\% $$ where \( \eta_{recycle} \) is the recycling efficiency, \( M_{recovered} \) is the mass of recovered materials, and \( M_{input} \) is the input mass.
Turning to the impact on the lithium battery industry, I foresee substantial disruptions across the value chain. Cathode materials may see incremental changes, as existing nickel-manganese-cobalt (NMC) or lithium iron phosphate (LFP) chemistries can be adapted for solid state batteries with minimal modifications. However, anode materials are poised for a revolution. Graphite, the dominant anode material in liquid batteries, has a theoretical capacity of 372 mAh/g, whereas silicon-based anodes in solid state batteries can exceed 2000 mAh/g. This shift is driven by the equation for specific capacity: $$ C_s = \frac{nF}{M} $$ where \( C_s \) is the specific capacity in mAh/g, \( n \) is the number of electrons, \( F \) is Faraday’s constant, and \( M \) is the molar mass. Lithium metal anodes, with a theoretical capacity of 3860 mAh/g, offer even greater potential but require solutions for dendrite suppression.
Electrolytes and separators will experience the most dramatic changes. In liquid lithium-ion batteries, the electrolyte consists of organic solvents and lithium salts, while separators prevent short circuits. In solid state batteries, the solid electrolyte eliminates the need for liquid components and, eventually, separators. This transition can be summarized in the table below:
| Component | Role in Liquid Lithium-ion Batteries | Role in Solid State Batteries |
|---|---|---|
| Electrolyte | Liquid medium for ion transport | Solid medium (e.g., oxide, sulfide) |
| Separator | Physical barrier between electrodes | Often integrated or eliminated |
| Anode | Graphite-based | Silicon or lithium metal-based |
| Cathode | NMC, LFP, etc. | Similar, with possible enhancements |
This evolution is not just technical; it imbues industry players with a sense of urgency. Companies are accelerating R&D, forming alliances, and exploring vertical integration to secure their positions. The race to commercialize solid state batteries is fostering innovation in manufacturing techniques, such as roll-to-roll processing for solid electrolyte films. Furthermore, the push for sustainability is driving efforts to optimize resource use and develop circular economy models. In my opinion, the integration of AI and machine learning in materials discovery will play a pivotal role in overcoming bottlenecks. For instance, generative models can predict new solid electrolyte compositions with high ionic conductivity, accelerating the development of next-generation solid state batteries.
To address these challenges, I propose a multi-faceted approach. First, robust policy frameworks are essential to guide the growth of the solid state battery industry. This includes funding for basic research, tax incentives for production, and standards for safety and performance. Second, collaborative platforms that bring together academia, industry, and government can pool resources to tackle technical hurdles. For example, consortia focused on interface engineering could yield breakthroughs in reducing resistance. Third, international cooperation on supply chain security and recycling protocols will mitigate risks and promote sustainability.
In conclusion, as I reflect on the journey of solid state batteries, I am struck by their potential to revolutionize energy storage. While obstacles remain, the relentless pace of innovation gives me confidence that solid state batteries will eventually overcome these hurdles. The transition may be gradual, with semi-solid variants bridging the gap, but the destination—a world powered by safe, high-energy-density solid state batteries—is within reach. Through continued investment and collaboration, I believe we can unlock the full promise of this technology, reshaping industries and contributing to a sustainable energy future. The narrative of solid state batteries is still unfolding, and I am excited to witness its next chapters.
